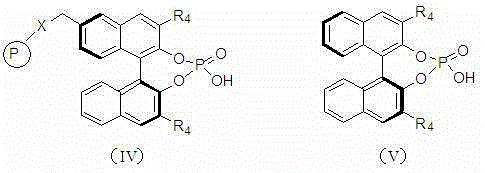
Preparation method for (S)-1,4-dihydropyridine calcium ion antagonist
A technology of calcium dihydropyridine and antagonists, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve problems such as cumbersome operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of (S)-1,4-dihydropyridine-2,6-dimethyl-4-(2-nitrophenyl)piperidine-3,5-dicarboxylic acid isobutylmethyl ester (1)
[0029] The structure of compound (1) and catalyst (1) used is shown in the following formula:
[0030]
[0031] N 2 Under protection, in the reactor, add 2.33g (0.01moL) 2-(2-nitrobenzylidene) methyl acetoacetate, catalyst (1) 0.5g (1 mmoL, Merrifield resin chiral phosphoric acid content 2mmol / g) and 20 mL of acetonitrile, stirred at room temperature for 10 minutes, added 1.72 g (0.011 moL) of isobutyl β-aminocrotonate, heated to 50°C, and reacted for 24 hours. Cool, add 1 mL of water to quench the reaction, filter with suction, wash the catalyst with 3 mLх3 acetonitrile, dry, recover the catalyst, and recycle it. The washing solution and the reaction solution were combined, the solvent was spinned out, the residue was added with 10mL of water, extracted with 10mLх3 chloroform, dried, filtered with suction, and evaporated to dryness to o...
Embodiment 2
[0033] Preparation of (S)-1,4-dihydropyridine-2,6-dimethyl-4-(3-nitrophenyl)piperidine-3,5-dicarboxylic acid ethylmethyl ester (2)
[0034] The structure of compound (2) and catalyst (2) used is shown in the following formula:
[0035]
[0036] N 2 Under protection, add 2.33g (0.01moL) methyl 2-(3-nitrobenzylidene)acetoacetate, catalyst (2) 0.77g (1 mmol) and 21mL chloroform into the reactor, and stir at room temperature for 10 minutes , add 1.29 g (0.011moL) ethyl β-aminocrotonate, heat to 50°C, and react for 24 hours. Cool, and use 6mL of 2mol L for the reaction solution -1 Adjust the pH of the NaOH solution to 11, separate layers, extract the aqueous phase with 10mLх3 chloroform, dry, filter with suction, evaporate the solvent to obtain a solid, and recrystallize the solid with ethanol-water (w / w=95:5) to obtain light yellow Compound (2). Yield 87%, ee value 91%, [a] D 25 = +13.6°(c = 0.5, C 2 h 5 OH), mp: 158-159°C. For the aqueous phase, use 6mL of 2mol·L -1...
Embodiment 3
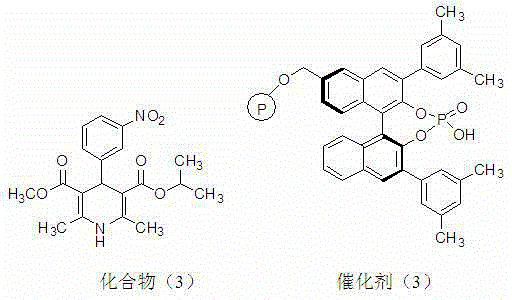
[0038] (S)-1,4-Dihydropyridine-2,6-dimethyl-4-(3-nitrophenyl)piperidine-3,5-dicarboxylate isopropylmethyl ester (3) preparation
[0039] The structure of compound (3) and the structure of catalyst (3) are shown in the following formula:
[0040]
[0041] N 2 Under protection, in the reactor, add 2.33g (0.01moL) 2-(3-nitrobenzylidene) methyl acetoacetate, catalyst (3) 0.45g (1 mmolL, chiral phosphoric acid content in Merrifield resin 2.2 mmol / g) and 20mL acetonitrile, stirred at room temperature for 10 minutes, added 1.56 g (0.011moL) isopropyl β-aminocrotonate, heated to 50°C, and reacted for 24 hours. Cool, add 1 mL of water to quench the reaction, filter with suction, wash the catalyst with 3 mLх3 acetonitrile, dry, recover the catalyst, and recycle it. Combine the washing solution and the reaction solution, spin out the solvent, add 10mL of water to the residue, extract with 10mLх3 chloroform, dry, filter with suction, evaporate the solvent to dryness, and obtain a so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com