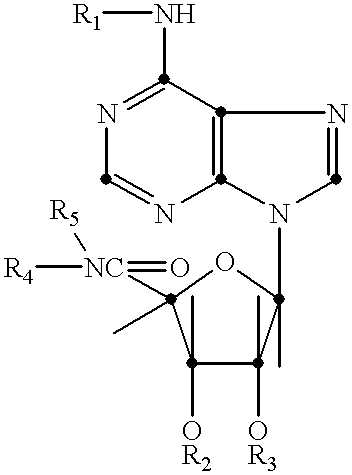
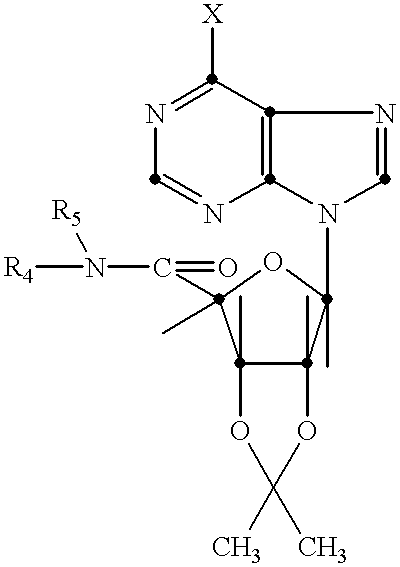
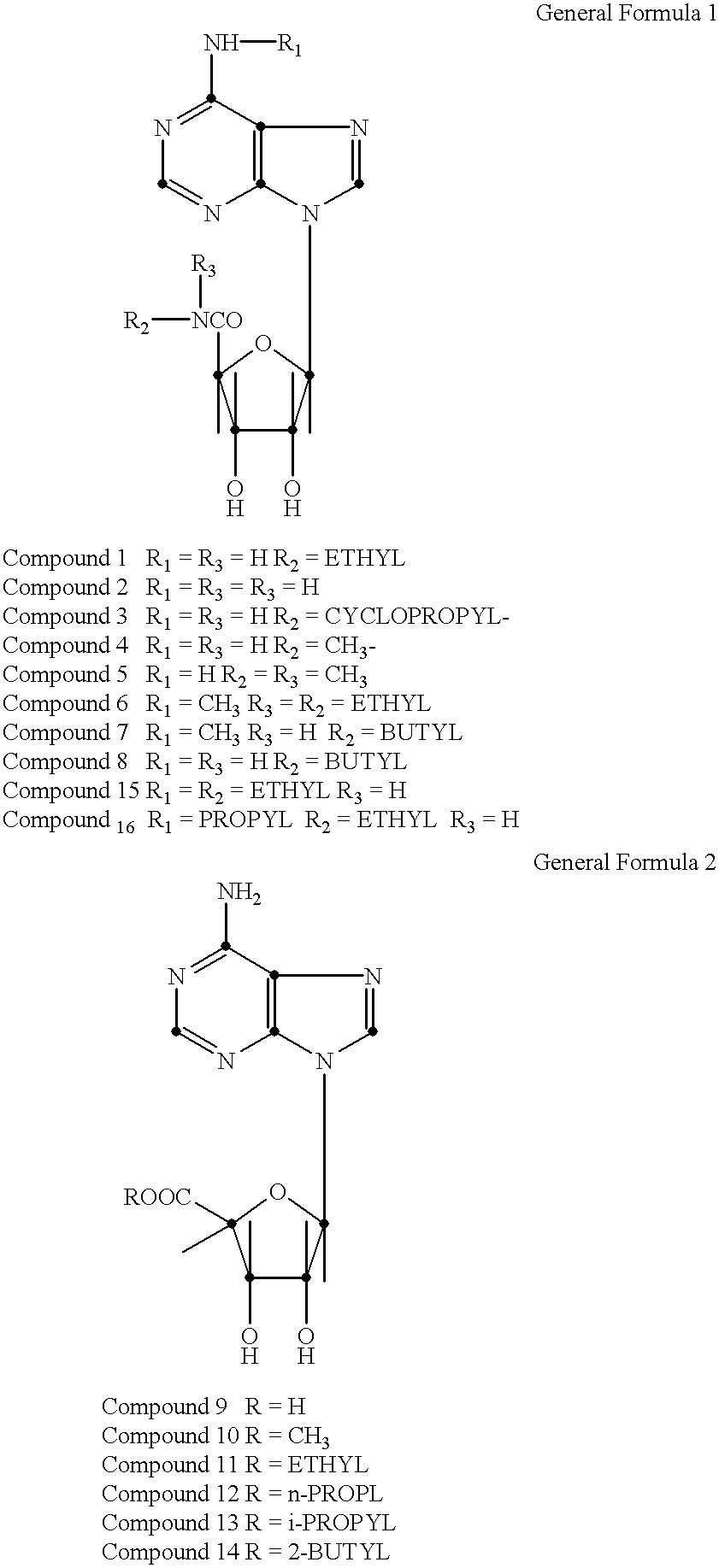
N-6 substituted-5'-(N-substituted carboxamido)adenosines as cardiac vasodilators and antihypertensive agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific examples
Ethyl N.sup.6 -(3-pentyl)adenosine-5'-uronamide, (Compound 17) mp 176-177, uv .lambda.max(.epsilon.)=269 nm(18.1.times.10.sup.3) at pH 7. Anal. Calculated for C.sub.17 H.sub.26 N.sub.6 O.sub.4 (378.44): C, 53.96; H, 6.93; N, 22.21. Found: C, 53.82; H, 6.95; N, 22.17. Molar potency ratio (mpr) 3.3.+-.0.25; anti-hypertensive activity at 0.05 mg / kg (22,20,22).
Ethyl N.sup.6 -cyclohexyladenosine-5'-uronamide, (Compound 18) mp 133-135; uv.lambda.max(.epsilon.)=270(18.8.times.10.sup.3) at pH 7. Anal. Calculated for C.sub.18 H.sub.26 N.sub.6 O.sub.4 (382.38): C, 55.37; H, 6.71; N, 21.52. Found: C, 55.34; H, 6.86; N, 21.42. Molar potency ratio (mpr) 1.5.+-.0.24; anti-hypertensive activity at 0.1 mg / kg (13,17,15).
Ethyl N.sup.6 -(S)-1-phenyl-2-butyladenosine-5'-uronamide, (Compound 19) mp 177-179; uv .lambda.max(.epsilon.)270 nm(19.2.times.10.sup.3) at pH 7; .alpha..sub.D.sup.25 =+27 c=1 in 95% ETOH. Anal. Calculated for C.sub.22 H.sub.28 N.sub.6 O.sub.4 (440.51): C, 59.99; H, 6.41; N, 19.08. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com