Brown planthopper VgR polypeptide and multi-resistant preparation method thereof
A technology for antibody preparation and anti-BPH, applied in chemical instruments and methods, specific peptides, anti-animal/human immunoglobulins, etc., can solve the problems of no commercial products and limited in-depth research on the biological functions of insect VgR proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
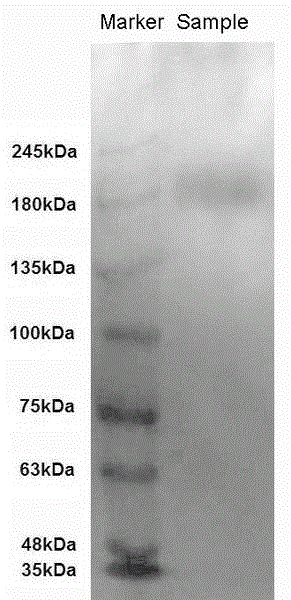
Image
Examples
Embodiment 1
[0015] Example 1: Analysis of the VgR sequence of the brown planthopper and the design and synthesis of the VgR polypeptide
[0016] According to the N. lugens VgR sequence on GenBank (accession number: ADE34166), it is known that the VgR protein sequence of N. lugens contains 1931 amino acids. Using the Protean program module in the DNAstar software to analyze the characteristics of the N. lugens VgR protein, it is known that the molecular weight of the protein is 215198.74 dahl Dayton, the isoelectric point is 4.95, which is an acidic protein. After further analyzing the characteristics of the protein's amino acid sequence, such as antigenicity, hydrophilicity, and surface possibility, a polypeptide sequence with the sequence RKGNADQSVATKSD was screened out and suitable for use as an antigen (1295aa—1308aa ). In order to facilitate cross-linking with the carrier protein and increase the immunogenicity of the polypeptide, a cysteine C was added to the N-terminus of the abov...
Embodiment 2
[0017] Example 2: Cross-linking of polypeptides and carrier proteins
[0018] Use MBS as a cross-linking agent to cross-link the carrier protein KLH with the synthetic polypeptide: dissolve KLH with cross-linking buffer to a concentration of 10 mg / mL; dissolve MBS in DMF to a concentration of 10 mg / mL; dissolve the dissolved KLH solution and MBS solution by Mix at a ratio of 10:1 (W / W), activate KLH at room temperature for 30 minutes; purify the activated KLH solution with Sephadex G‐25; mix the activated KLH solution with the peptide solution at a ratio of 1:1 (W / W), at room temperature The reaction was carried out for 3 hours; the above reaction solution was dialyzed in PBS at 4° C. overnight to obtain the polypeptide-KLH complex protein.
Embodiment 3
[0019] Embodiment three: the immunization of experimental animal
[0020] New Zealand male rabbits of appropriate age were selected as immunized animals, and 2-3 mL of blood was collected from the ear vein before immunization, which was used as a negative control for subsequent ELISA detection. For the first immunization, 0.5 mg of polypeptide-KLH complex protein was dissolved in 0.5 mL of PBS solution, fully mixed with an equal volume of Freund's complete adjuvant to emulsify, and injected subcutaneously into rabbits at multiple points. Two weeks later, the first booster immunization was carried out. Dissolve 0.5 mg of polypeptide-KLH cross-linked complex protein in 0.5 mL of PBS solution, fully mix and emulsify with an equal volume of Freund's incomplete adjuvant, and inject subcutaneously at multiple points. The booster immunization with the same operation was carried out every 3 weeks, a total of 3 times before and after. One week after each booster immunization, a small ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com