Method for extracting and separating trivalent lanthanum and trivalent actinium ion
A technology of actinides and lanthanides, applied in the field of nuclear fuel cycle and waste liquid treatment, can solve the problems of decreased extraction and separation capacity, deviation from the ideal range, unfavorable continuous extraction operation, etc., and achieve high-efficiency extraction and separation performance and good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
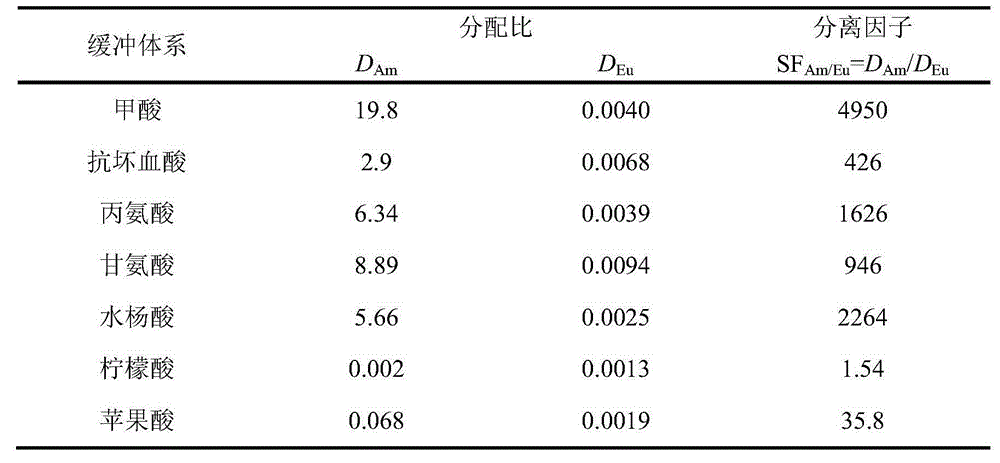
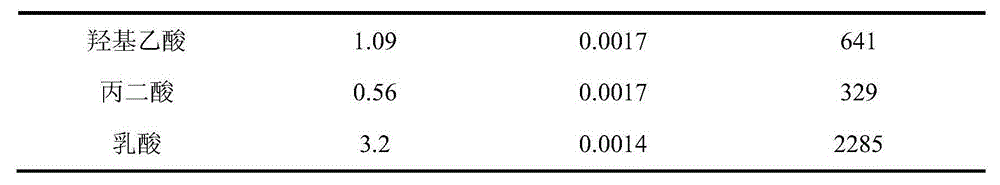
[0025] Example 1: Extraction and separation of Am(III) and Eu(III) / Nd(III) by HC301-n-dodecane. When performing extraction experiments, the aqueous phase contained trace amounts of 241 Am, tracer 151,152 Eu, 1.8mmol / L Eu(III), 14mmol / L Nd(III), the total concentration of formic acid-ammonium formate in the aqueous phase is 0.05mol / L, the pH of the aqueous phase is adjusted to 3.75, and the concentration of HC301 in the organic phase is 0.5 mol / L. After the two phases were mixed and stirred for 5 minutes, they were centrifuged, and the aqueous phase and the organic phase were taken for pH measurement, radioactive detection and ICP-MS measurement, and the extraction rate of Am(III) was determined to be 85%, and the extraction rate of Eu(III) was 0.03%, and the extraction rate of Nd(III) was 0.2%. The pH change of the aqueous phase before and after extraction is less than 0.02.
Embodiment 2
[0026] Example 2: HC301-kerosene extraction and separation of Am(III) and Eu(III) / Nd(III). When performing extraction experiments, the aqueous phase contained trace amounts of 241 Am, tracer 151,152Eu, 1.8mmol / L Eu(III), 14mmol / L Nd(III), the total concentration of formic acid-ammonium formate in the aqueous phase is 0.05mol / L, the pH of the aqueous phase is adjusted to 4.00, and the concentration of HC301 in the organic phase is 0.5 mol / L. After the two phases were mixed and stirred for 5 minutes, they were centrifuged, and the aqueous phase and the organic phase were taken for pH measurement, radioactivity detection and ICP-MS measurement respectively. It was determined that the extraction rate of Am(III) was 94%, and the extraction rate of Eu(III) was 0.1%, the extraction rate of Nd(III) was 0.7%. The pH change of the aqueous phase before and after extraction is less than 0.02.
Embodiment 3
[0027] Example 3: Extraction and separation of Am(III) and Eu(III) / Nd(III) by HC301-n-dodecane. When performing extraction experiments, the aqueous phase contained trace amounts of 241 Am, tracer 151,152 Eu, 1.8mmol / L Eu(III), 14mmol / L Nd(III), the total concentration of formic acid-sodium formate in the aqueous phase is 0.20mol / L, the pH of the aqueous phase is adjusted to 4.00, and the concentration of HC301 in the organic phase is 0.5mol / L. After the two phases were mixed and stirred for 5 minutes, they were centrifuged, and the aqueous phase and the organic phase were taken respectively for pH measurement, radioactive detection and ICP-MS measurement. It was determined that the extraction rate of Am(III) was 87%, and the extraction rate of Eu(III) was less than 0.1%. The pH change of the aqueous phase before and after extraction is less than 0.02.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com