A multi-component comprehensive quantification method for quality assessment and control of traditional Chinese medicine and its application
A technology of medicinal materials and content, which is applied in the direction of measuring devices, material separation, and analysis of materials, etc., can solve the problems of rough titer determination and inability to specifically reflect the laxative intensity of the ingredients that cause diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
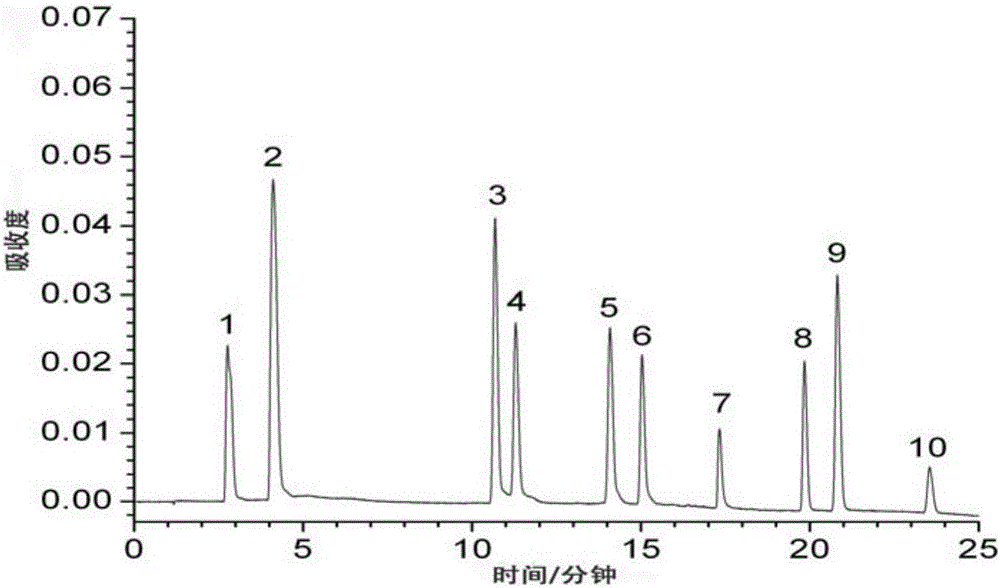
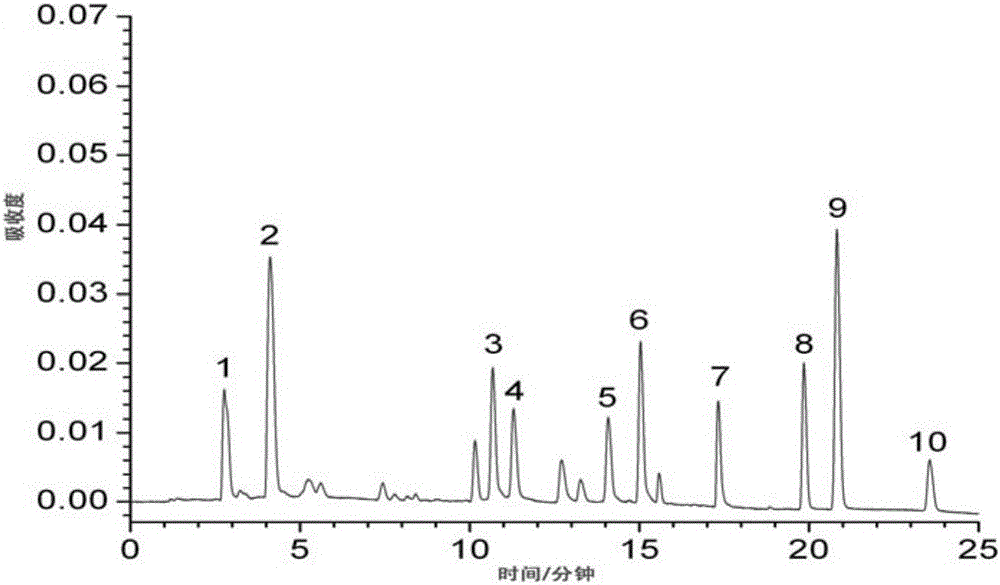
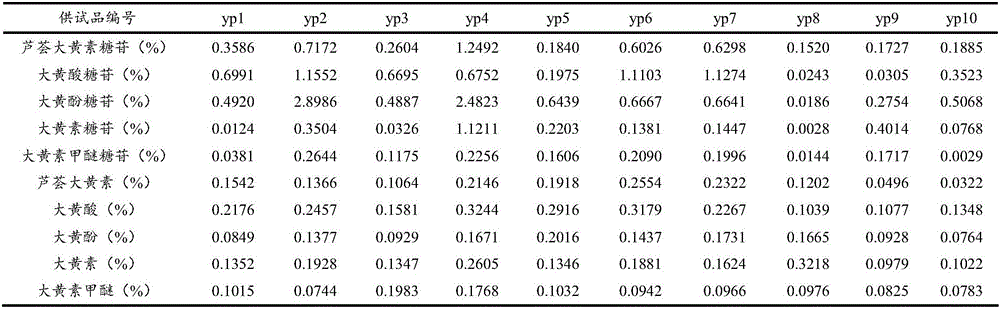
[0115] Embodiment 1 (measurement of content of 10 kinds of active ingredients such as aloe-emodin-8-o-β-D-glucoside in rhubarb medicinal materials)
[0116] Test product: rhubarb test product (that is, the sample of rhubarb medicinal material to be tested)
[0117] Reference substance: aloe-emodin-8-o-β-D-glucoside (batch number 141225, used for content determination as 98.78), rhein-8-o-β-D-glucoside (batch number 141211, for content determination 98.57%), chrysophanol-8-o-β-D-glucoside (batch number 141220, used for content determination in 99.42%), emodin-8-o-β-D-glucoside (batch number 141209 , used for content determination at 98.67%), emodin methyl ether-8-o-β-D-glucoside (batch number 141012, used for content determination at 99.14%), provided by Chengdu Croma Biotechnology Company;
[0118] Aloe-emodin (batch number 110795-201007, for content determination at 98.05%), rhein (batch number 110757-200206, for content determination at 98.65%), emodin (batch number 110756,...
Embodiment 2
[0134] Embodiment 2 (the content determination of sennoside A, sennoside B in rhubarb medicinal materials)
[0135] Test product: rhubarb test product (that is, the sample of rhubarb medicinal material to be tested)
[0136] Reference substances: Sennoside A (batch number 13122701, for content determination at 98.58%), sennoside B (batch number 13062606, for content determination at 98.35%), provided by Chengdu Purui Biotechnology Company;
[0137] Waters Acquity ultra-high performance liquid chromatography (Waters, USA), equipped with Waters PDADetector (diode array detector), automatic sampler, Empower 2 chromatographic workstation, AcquityUPLC BEH C18 column (1.7μm, 2.1 × 100mm), XS-205 electronic balance (METTLERTOLEDO), AL-204 electronic balance (METTLER TOLEDO), ultrasonic instrument (Nanjing Xinchen Biotechnology Co., Ltd., 40KHz).
[0138] Preparation of the reference solution of sennoside A and sennoside B:
[0139] with 0.1% NaHCO 3 45% methanol aqueous solution was...
Embodiment 3
[0145] Embodiment 3 (the detailed investigation of the mensuration of rhubarb-induced laxative effect component potency and the calculation of equivalence coefficient)
[0146] 140 ICR male mice, weighing 20g, were divided into 14 groups, 10 in each group. They were fasted for 12 hours before the experiment, and the first group was regarded as the normal group, without any modeling and administration; the second group As a model control group, only the compound difenoxate tablet was given to cause a constipation model; the remaining 12 groups were administered corresponding to 12 effect components. First use compound diphenoxylate tablet (dose 60mg·kg -1 ) for modeling by intragastric administration, and intragastric administration after 1 hour of modeling, with a dosage of 0.4ml (wherein the concentrations of 12 active ingredients such as aloe-emodin-8-o-β-D-glucoside and sennoside A are 1 mg / ml, dissolved in hot water at 37°C), after administration, fasting without water, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com