New application of N-(1-benzylpiperidine-4-amidogen)-2-(4-phenoxy benzoyl) acetamide
A technology of benzoylphenoxy and benzylpiperidine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
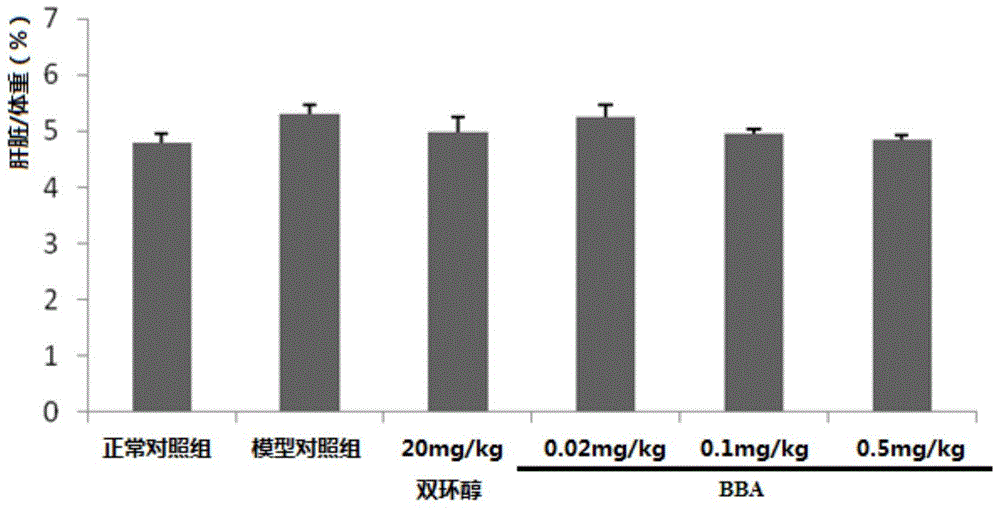
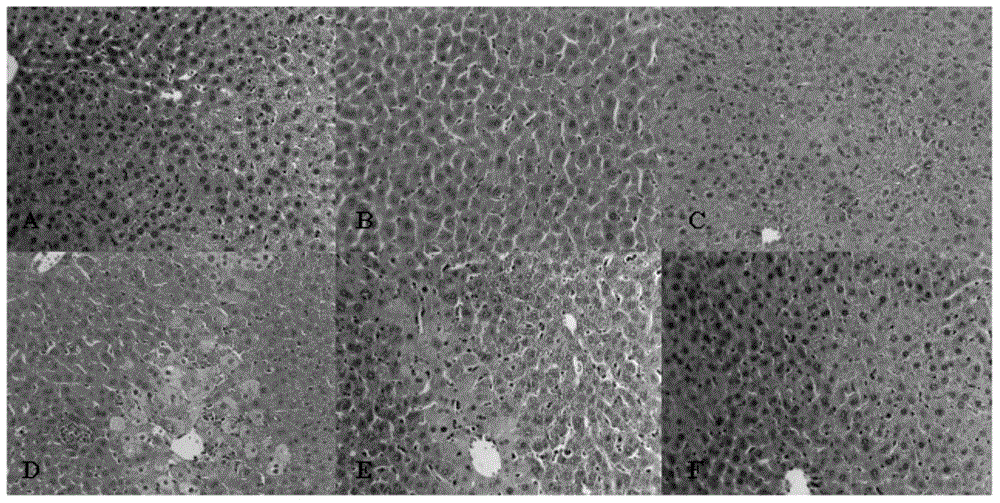
[0023] Example 1 N-(1-benzylpiperidin-4-yl)-2-(4-benzoylphenoxy)acetamide to CCl 4 Protective effect of induced acute liver injury in mice
[0024] 1. Test drugs
[0025] Drug dosage: N-(1-benzylpiperidin-4-yl)-2-(4-benzoylphenoxy)acetamide (BBA) high dose group is 0.5mg / kg, and the prepared concentration is 0.05mg / ml , administered by intragastric administration at 0.1ml / 10g; the middle dose group is 0.1mg / kg, the prepared concentration is 0.01mg / ml, administered by intragastric administration at 0.1ml / 10g; the low dose group is 0.02mg / kg, the prepared concentration is 0.002mg / ml, orally administered at 0.1ml / 10g.
[0026] Experimental control: normal saline (sodium chloride injection) was used for the blank control, administered by intragastric administration of 0.1ml / 10g; the positive control drug was bicyclol (Bicyclol, gifted by Professor Su Xianbin of Nanjing University of Technology), according to the clinical recommended dosage and Animal tolerance capacity, the de...
Embodiment 2
[0075] Example 2 Drugs for preventing and treating liver damage
[0076] The medicine is composed of N-(1-benzylpiperidine-4-amino)-2-(4-benzoylphenoxy)acetamide and a pharmaceutically acceptable carrier, and the dosage forms include tablets, capsules, Granules, pills, solutions, injections, sprays and aerosols.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com