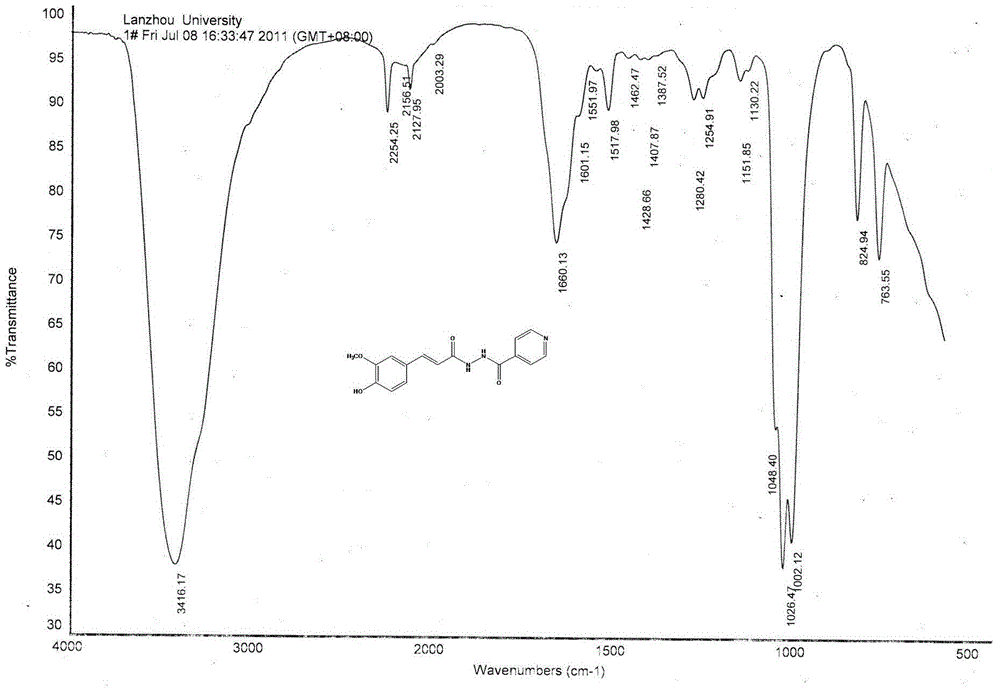
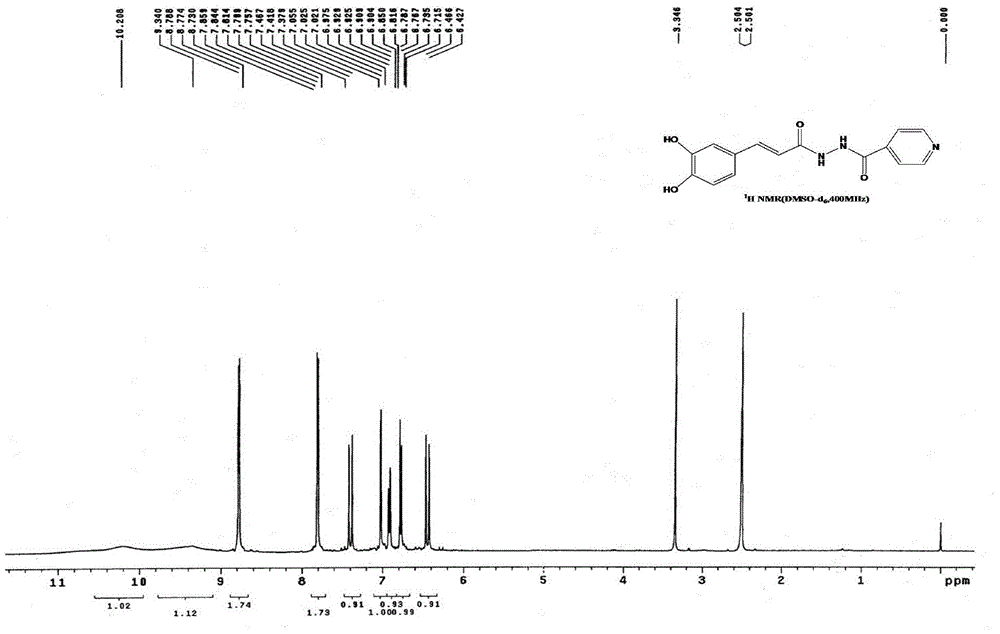
Isoniazid caffeic acid amidated derivative and application thereof in anti-mycobacterium tuberculosis drug
A technology of caffeic acid amide and isoniazid, which is applied in the field of medicine, can solve the problems of liver damage and inability to effectively inhibit drug-resistant Mycobacterium tuberculosis, and achieve high activity and the effect of inhibiting drug-resistant Mycobacterium tuberculosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of Isoniazid Caffeic Acid Amidated Derivatives
[0023] 1. Preparation of Caffeic Acid
[0024] Stir and reflux 0.02mol of 3,4-dihydroxybenzaldehyde, 0.03mol of malonic acid and an appropriate amount of catalyst at 118°C for 1 to 2 hours. TLC detects that the benzaldehyde disappears completely and the reaction is complete. After reflux finishes, carry out steam distillation, remove unreacted benzaldehyde. If the color is darker, add 2-3g of activated carbon, boil for a few minutes to decolorize, filter while it is hot, and add hydrochloric acid to the hot filtrate under stirring until it is obviously acidic (pH: 4-5). After cooling, after all the crystals are precipitated, filter with suction, wash with water, and dry to obtain the crude product. After separation and purification by column chromatography, the product was dried to obtain the target product caffeic acid (3,4-dihydroxycinnamic acid), which was weighed to calculate the yield.
[002...
Embodiment 2
[0031] Example two isoniazid caffeic acid amidated derivatives in vitro inhibition of drug-resistant Mycobacterium tuberculosis test
[0032] Experimental material: drug-resistant Mycobacterium tuberculosis (H 37 R v ), donated by Lanzhou Pulmonary Hospital, Gansu Province.
[0033] Middlebrook7H9 was provided by Becton, Dickinson and Company (BD), and other reagents in the medium were of domestic analytical grade.
[0034] Modified 7H9 medium formula: 10% azodicarbonamide (ADC), 0.05% Tween-80 and 0.5% glycerol, Middlebrook7H9 medium (distilled water 897.5ml, Middlebrook7H9 powder 4.7g, 100% glycerol 2ml , 100% Tween 800.5ml).
[0035] The present embodiment adopts the 2-fold dilution method to detect that the compound of the present invention is effective against drug-resistant Mycobacterium tuberculosis H 37 R v inhibitory effect.
[0036] First, the isoniazid caffeic acid amidated derivatives obtained in Example 1 were formulated into different concentrations of test...
Embodiment 3
[0041] The hydrolysis kinetic characteristic of embodiment three isoniazid caffeic acid amidated derivatives in blood plasma
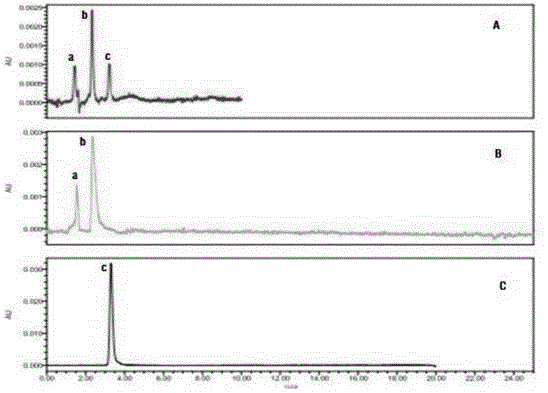
[0042] Adopt HPLC method to detect the hydrolysis kinetics characteristic of isoniazid caffeic acid amidated derivative of the present invention in blood plasma, method is: after compound is hydrolyzed in the blood plasma of 80%PBS (PH7.4) 12h, adopt C 18 The column is used for HPLC detection, detection conditions: the mobile phase is methanol-water (45:55), pH=3.5, the detection wavelength is 290nm, the column temperature is 25°C; the injection volume is 10μL, and the flow rate is 1.0mL·min -1 , while using caffeic acid as a reference substance for hydrolysis kinetics detection, the results are shown in Figure 5 . Wherein A is the HPLC figure measured after the PBS buffer solution of the amidated derivative of isoniazid caffeic acid is hydrolyzed in plasma, B is the HPLC figure measured by the PBS buffer solution of the reference substance isoniazid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com