Quantitative detection method of tea moth nucleopolyhedrosis virus
A quantitative detection method, nuclear polyhedron technology, applied in the direction of microorganism-based methods, microorganism measurement/inspection, biochemical equipment and methods, etc., can solve the problems of difficult effective monitoring of preparation quality and extensive toxicity index detection, etc. To achieve fine detection of toxicity indicators, to solve accurate qualitative problems, and to shorten the cycle of toxicity evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Irfa Obtaining NPV Virus Samples and Extracting Genomic DNA
[0023] Infect the tea moth larvae with the tea moth nuclear polyhedrosis virus, collect the carcasses, and use PBS solution as the buffer solution to homogenize repeatedly. The supernatant is the crude virus extract; the crude virus extract is centrifuged at 35000g for 2 hours, and the precipitate is the purified virus particles; the purified virus particles are washed repeatedly with 1×PBS until milky white; use TaKaRa MiniBEST Viral RNA / The DNA Extraction KitVer.5.0 kit was used for the extraction of the genome of the tea moth nucleopolyhedrosis virus. The steps are as follows: 1) Lysis of the virus ① Take 200 μl of the virus stock solution. ② Add 200 μl of Buffer VGB, 20 μl of Proteinase K and 1.0 μl of CarrierRNA, mix well and incubate in a 56°C water bath for 10 minutes. ③Centrifuge, take the supernatant; add 200μl of absolute ethanol to the supernatant, and mix well by pipetting. 2) Pla...
Embodiment 2
[0024] Embodiment 2: target fragment PCR amplification
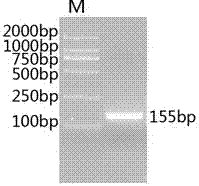
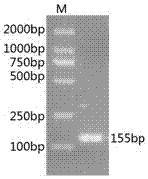
[0025] according to Irfa NPV genome sequence (FJ362523) Lef 11 genes, using DNAStar software, designed a pair of specific primers Irfa NPV Lef 11F and primers Irfa NPV Lef 11R, synthesized by Shanghai Sangon Biotechnology Co., Ltd., the primer sequences are: 5'- ACAATGAGAGTGGACCTTACGA-3' (shown in SEQ ID NO: 1) and 5'-ACGCGCTAAAACACGATATGA -3' (shown in SEQ ID NO: 2 shown), amplified Lef 11. PCR reaction uses 25 µL system (template 2 µL, 10×buffer 2.5 µL, dNTP 2 µL, forward and reverse primers 0.5 µL, Tag enzyme 0.25 µL, add sterilized double distilled water to 25 µL), PCR amplification uses two-step method, The procedure is as follows: firstly, pre-denaturation at 94°C for 3min; then 30s at 94°C, 45s at 60°C; 35 cycles, and finally extension at 72°C for 7min. Take 10ul of the PCR product and test it by 1% agarose gel electrophoresis. figure 1 , which is consistent with the expected size. Finally, use th...
Embodiment 3
[0026] Embodiment 3: the preparation of plasmid standard and its concentration determination
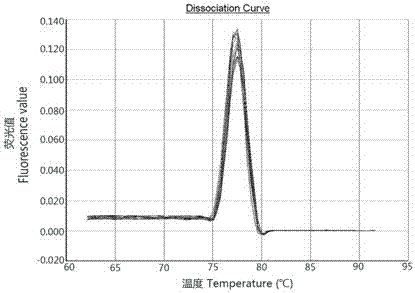
[0027]The amplified in embodiment 2 Lef The 11 gene fragments were connected with the vector pGEM-T, transformed into Escherichia coli DH5a, single-clonal strains were picked, and the bacteria were shaken. Use the kit to extract single colony plasmid DNA and carry out PCR identification. The recombinant plasmids identified as positive were sent to Shanghai Sangon Biotechnology Co., Ltd. for sequencing. Compare with the reference sequence to confirm the construction result. After the construction is completed, measure its concentration with a UV spectrophotometer, and dilute 1 µL of positive plasmid 100 times to measure its OD 260 and OD 280 value, calculate the recombinant plasmid concentration and OD 260 / OD 280 Ratio, the recombinant plasmid was diluted to 10 -10 copies / µL, stored in a -20°C freezer.
[0028] For the results of PCR identification of the recombinant plasmid,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com