Sodium prasterone sulfate sustained-release capsule and preparation method thereof
A technology of pratestosterone sulfate sodium and sustained-release capsules is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., which can solve the problems that need to be deepened, and achieve the effects of improving safety and reducing toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
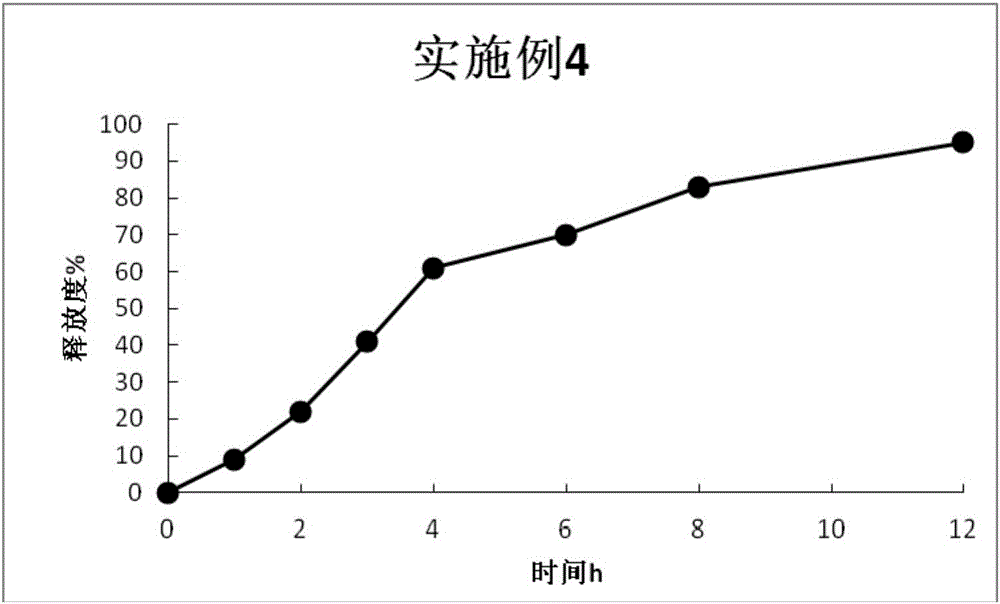
Examples
Embodiment 1
[0052] In this embodiment, the drug-loaded pellet core was prepared according to the prescription shown in Table 1 below and the operation steps described below:
[0053] Preparation:
[0054] Pass prasterone sulfate sodium through an 80-mesh sieve, weigh the sieved prasterone sulfate sodium and the blank ball core according to the prescription amount shown in Table 1, and use a centrifugal granulator and a fluidized bed to granulate respectively. After the granulation is completed, the drug-loaded pellet core is obtained.
[0055] Table 1
[0056]
[0057] Investigation on the preparation process of drug-loaded pellet core:
[0058] The preparation of drug-loaded pellet cores by fluidized bed and centrifugal granulator was investigated, and the content uniformity of drug-loaded pellet cores prepared by the two methods was compared. The results are shown in Table 2.
[0059] Table 2
[0060] Preparation
centrifugal granulator
conte...
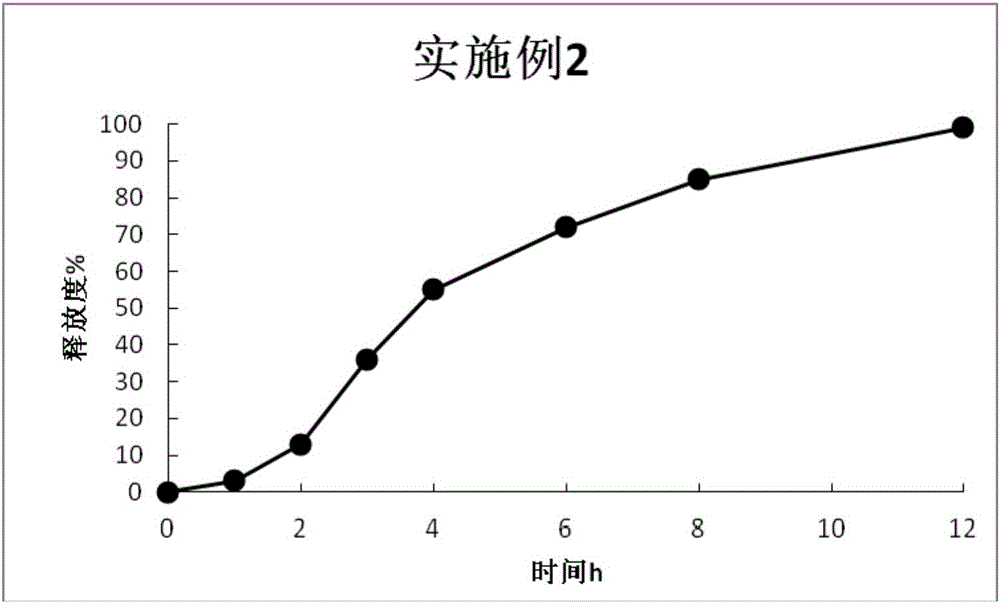
Embodiment 2
[0066] ①Preparation of drug-loaded pellet core
[0067] prescription:
[0068] Prasterone Sulfate Sodium 200g
[0069] Blank ball core (sucrose ball core) 274g
[0070] 8% PVP-k30 solution (solvent is 90% ethanol) 150g
[0071] Preparation Process:
[0072] Pass prasterone sulfate sodium through an 80-mesh sieve, weigh the prescription amount, and pour it into the hopper of the centrifugal granulator. Pour blank pellet cores into a centrifugal granulator for granulation, set the air inlet pressure to 0.7 bar, atomization pressure to 2 bar, thimble pressure to 1 bar, feeding speed to 3 rpm, peristaltic pump efficiency to 10%, and turntable speed to 135rpm, spray 8% PVP-k30 solution (the solvent is 90% ethanol), and the drying temperature is 55°C. After the granulation is finished, about 480 g of drug-loaded pellet cores are obtained.
[0073] ②Drug-loaded pill core sustained-release layer coating
[0074] prescription:
[0075]
[0076] Preparation Process:
[0077]...
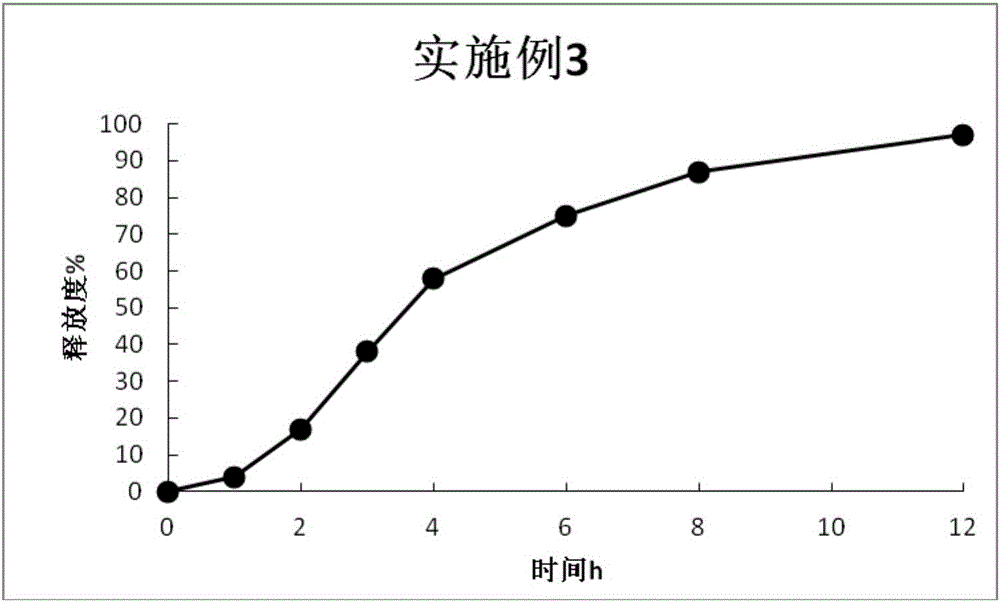
Embodiment 3
[0080] ①Preparation of drug-loaded pellet core
[0081] prescription:
[0082] Prasterone Sulfate Sodium 200g
[0083] Blank ball core (sucrose ball core) 274g
[0084] 8% PVP-k30 solution (solvent is 90% ethanol) 150g
[0085]Preparation Process:
[0086] Pass prasterone sulfate sodium through an 80-mesh sieve, weigh the prescription amount, and pour it into the hopper of the centrifugal granulator. Pour the blank pellet core into the centrifugal granulator for granulation, set the air inlet pressure to 0.7bar, atomization pressure to 2bar, thimble pressure to 1bar, feeding speed to 3rpm, peristaltic pump efficiency to 10%, turntable speed to 135rpm, spray 8% PVP -k30 solution (solvent is 90% ethanol), dried at 55°C. After the granulation is finished, about 480 g of drug-loaded pellet cores are obtained.
[0087] ②Drug-loaded pill core sustained-release layer coating
[0088] prescription:
[0089]
[0090] Preparation Process:
[0091] Add the prescribed amount o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com