Application of 5alpha-androstane-3beta,5,6beta-triol and analogues thereof in prevention and treatment of altitude disease caused by hypobaric hypoxia
A technology of hypobaric hypoxia and analogs, used in medical preparations containing active ingredients, drug delivery, blood diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
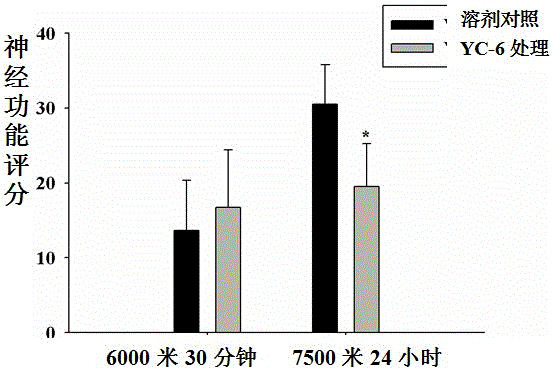
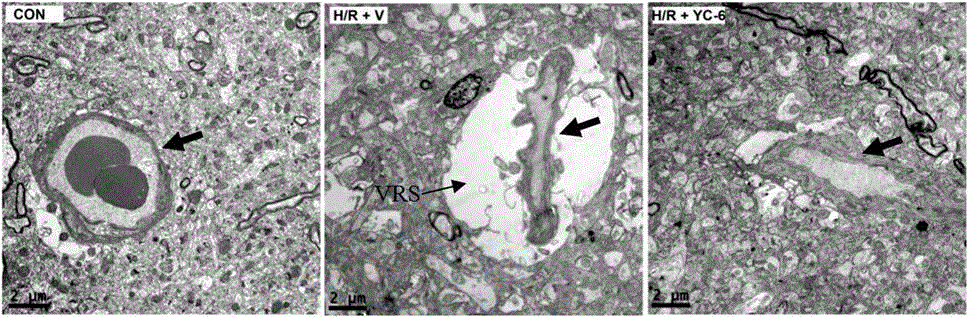
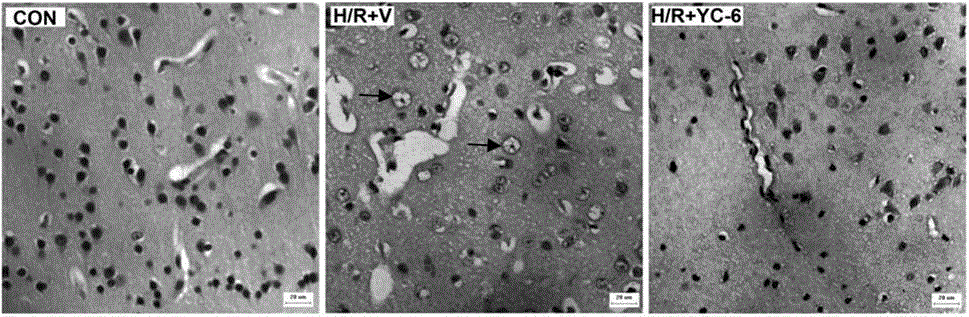
[0029] Further explain the present invention by specific embodiment below, but the present invention is not limited in the embodiment.
[0030] Verification of new application of 5α-androst-3β,5,6β-triol
[0031] 1. Animals: 17 healthy male cynomolgus monkeys (Macaca fascicularis), aged 6 to 6.5, weighing 6.8-7.5Kg. The use of experimental animals was approved by the Experimental Animal Management and Use Committee and the Experimental Animal Ethics Committee, and the experimental protocol complied with the principles and regulations of animal protection, animal welfare and ethics.
[0032] Seventeen male cynomolgus monkeys were randomly divided into 3 groups (Table 1):
[0033] Table 1: Explanation of groups of experimental animals
[0034]
[0035] 2. Main instruments and parameters: Plateau environment simulation hypobaric chamber group is a low-temperature and low-pressure experimental platform system for simulating plateau environment. It can automatically control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com