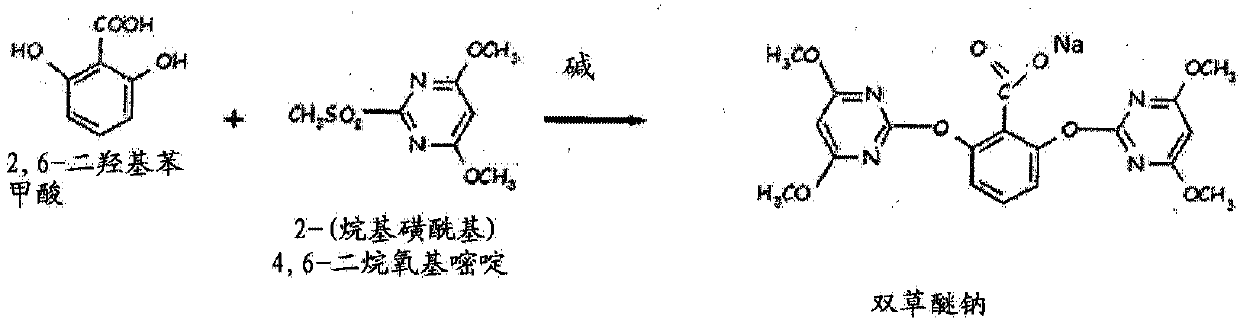
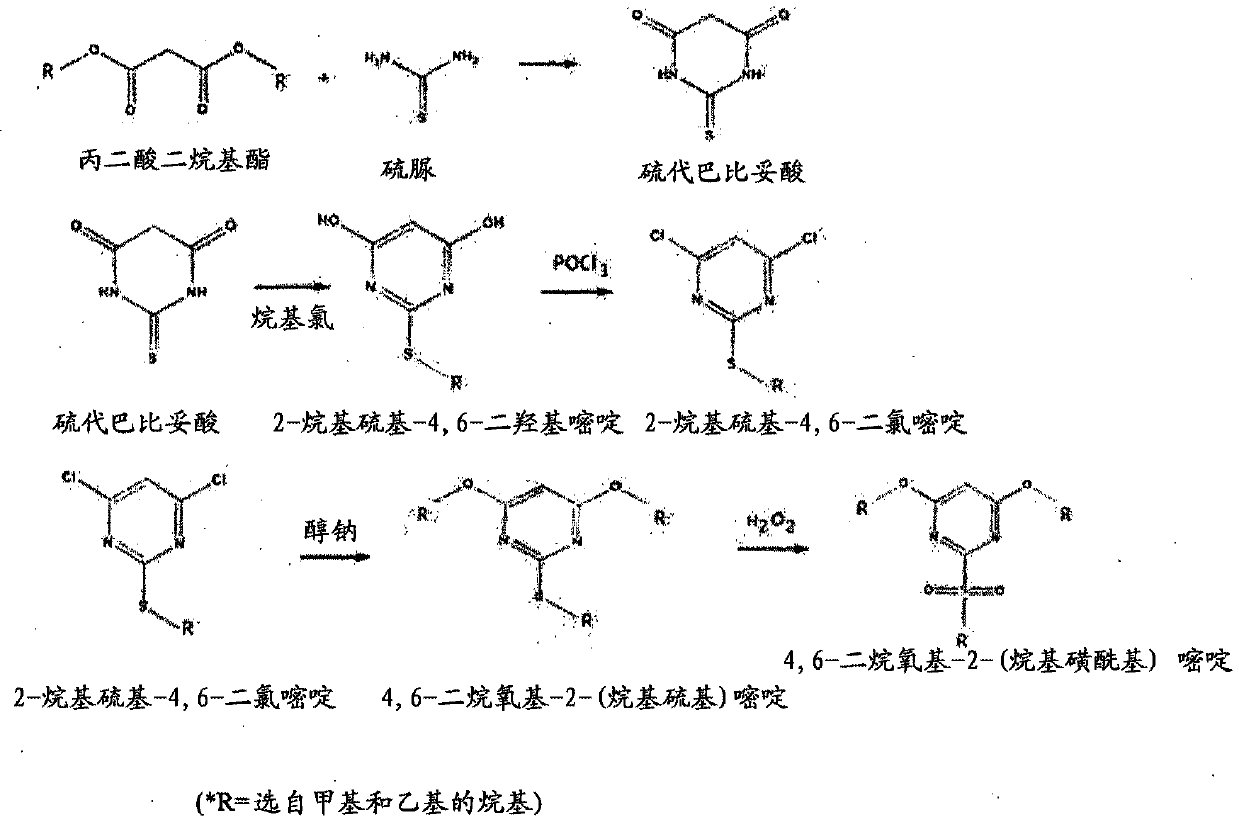
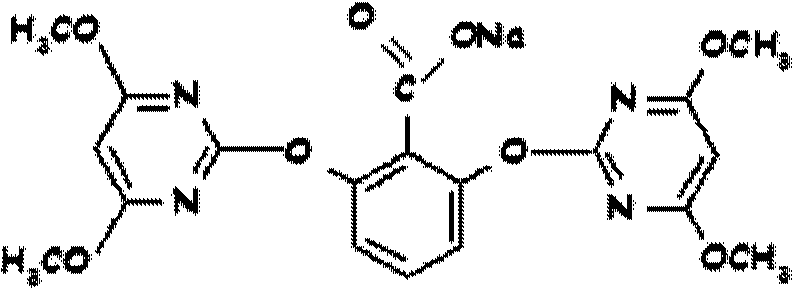
Method for preparing bispyribac sodium and its intermediates
A technology for bispyribacin sodium and sodium salt, which is applied in the field of preparing bispyribacin sodium and its intermediates, and can solve the problems of low yield, slow reaction rate, high impurity content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] A clear solution of 154 g of 2,6-dihydroxybenzoic acid in 500 ml of dimethyl sulfoxide was added to 130 g of a commercially available solution dissolved in 2 liters of dimethyl sulfoxide over 2-3 hours at 30-32°C. A mixture of sodium hydride (60% oil-based emulsion). The material was further stirred at 30-32°C for 2 hours, and 480 grams of 4,6-dimethoxy-2-(methylsulfonyl)pyrimidine was added over 2 hours. The reaction is maintained at 30-32°C until complete to obtain bispyribac-sodium. The reaction was monitored by high performance liquid chromatography (HPLC). The material was filtered and washed with dimethylsulfoxide (DMSO). Add 1000ml of methanol to the wet solid again to form a slurry, and then add 1000ml of 75% aqueous methanol to form a slurry. The wet solid was dried to obtain 435 g of bispyribac-sodium. The sodium bispyribac was re-slurried in 1200 ml of toluene at reflux temperature, cooled, filtered and dried to obtain 385 g of sodium bispyribac (HPLC pur...
Embodiment 2
[0133] A clear solution of 154 g of 2,6-dihydroxybenzoic acid in 500 ml of dimethyl sulfoxide was added to 130 g of commercially available hydrogenate dissolved in 1500 ml of dimethyl sulfoxide at 30-32°C for 2-3 hours. Sodium (60% Oil Based Emulsion). The material was further stirred at 30-2°C for 2 hours, and 480 grams of 4,6-dimethoxy-2-(methylsulfonyl)pyrimidine was added over 2-3 hours. The reaction is maintained at 30-32°C until complete to obtain bispyribac-sodium. The reaction was monitored by high performance liquid chromatography. The material was filtered and washed with dimethylsulfoxide. Add 1000ml of methanol to the wet solid again to form a slurry, and then add 1000ml of 75% aqueous methanol to form a slurry. The wet solid was dried to obtain 430 g of bispyribac-sodium. The sodium bispyribac was re-slurried in 1200 ml of toluene at reflux temperature, cooled, filtered and dried to obtain 380 g of sodium bispyribac (HPLC purity 98.3%).
Embodiment 3
[0135] A clear solution of 154 g of 2,6-dihydroxybenzoic acid in 500 ml of dimethyl sulfoxide was added at 30-32° C. to 130 g of commercially available sodium hydride (60% oleyl sulfoxide) dissolved in 1500 ml of dimethyl sulfoxide. emulsion) mixture. The mass was stirred for a further 2 hours at 30°C and a slurry of 480 grams of 4,6-dimethoxy-2-(methylsulfonyl)pyrimidine in 750 ml of dimethylsulfoxide was added. The reaction is maintained at 30-32°C until complete to obtain bispyribac-sodium. The material was filtered and washed with dimethylsulfoxide. Add 1000ml of methanol to the wet solid again to form a slurry, and then add 1000ml of 75% aqueous methanol to form a slurry. The wet solid was dried to obtain 440 g of bispyribac-sodium. The sodium bispyribac was re-slurried in 1200 ml of toluene at reflux temperature, cooled, filtered and dried to obtain 380 g of sodium bispyribac (HPLC purity 98.5%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com