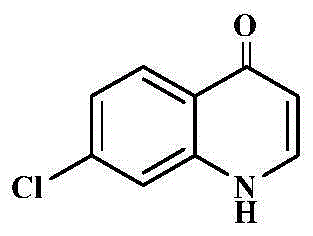
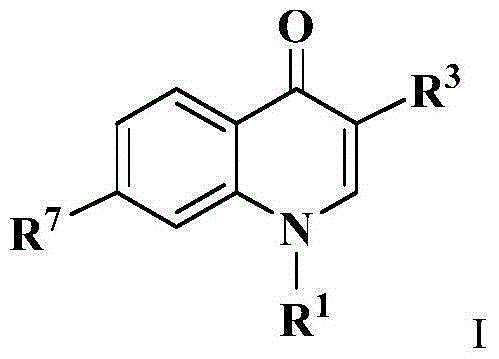
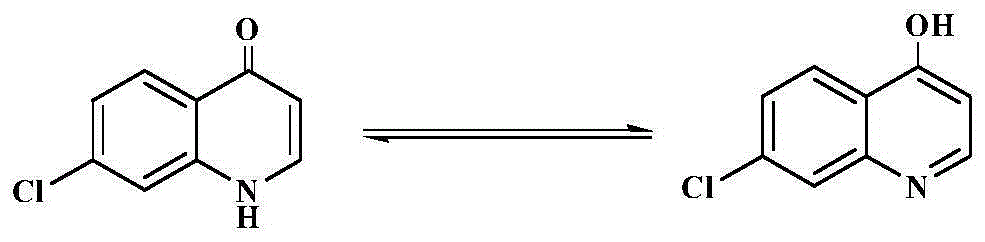Antibacterial chloroxoquinoline derivative
A technology of oxoquinoline and quinoline, applied in the field of chloroquine derivatives, can solve problems such as difficult to effectively eradicate Helicobacter pylori infection, failure of Helicobacter pylori eradication treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1: Preparation of 7-fluoro-1-methyl-4-oxo-quinoline (compound 7a)
[0206] Starting from 7-fluoro-4(1H)-oxoquinoline 6a (5.0 mmol) and iodomethane (7.5 mmol). A white solid (0.86 g, 86%) was obtained. ESI-MSm / z178[M+H] + . 1 HNMR (300MHz, CDCl 3 ):δ8.40-8.45(1H,m),7.47(1H,d,J=7.5Hz),7.00-7.11(2H,m),6.20(1H,d,J=7.5Hz),3.74(3H, s).
Embodiment 2
[0207] Embodiment 2: Preparation of 7-chloro-1-methyl-4-oxo-quinoline (compound 7b)
[0208] Starting from 7-chloro-4(1H)-oxoquinoline 6b (5.0 mmol) and iodomethane (7.5 mmol). A white solid (0.67g, 69%) was obtained, mp 233-234°C. ESI-MSm / z194[M+H] + . 1 HNMR (300MHz, CDCl 3 ): δ8.30 (1H, d, J = 8.7Hz), 7.43 (1H, d, J = 7.8Hz), 7.34 (1H, d, J = 1.8Hz), 7.28 (1H, dd, J = 8.7Hz ,J=1.8Hz),6.18(1H,d,J=7.8Hz),3.74(3H,s). 13 CNMR (75MHz, CDCl 3 )δ177.6 (C=O), 144.1, 141.4, 138.8, 128.8, 125.5, 124.5, 115.4, 110.8, 41.0.
Embodiment 3
[0209] Example 3: Preparation of 7-bromo-1-methyl-4-oxo-quinoline (compound 7c)
[0210] Starting from 7-bromo-4(1H)-oxoquinoline 6c (5.0 mmol) and iodomethane (7.5 mmol). A white solid (1.0 g, 88%) was obtained. ESI-MSm / z239[M+H] + . 1 HNMR (300MHz, CDCl 3 ):δ8.27(1H,d,J=8.7Hz),7.44-7.54(3H,m),6.23(1H,d,J=7.8Hz),3.76(3H,s). 13 CNMR (75MHz, CDCl 3 )δ176.5, 146.1, 128.5, 128.4, 127.1, 126.6, 126.0, 120.0, 109.9, 40.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com