Composition and application of iron-based heat source for non-combustible cigarettes based on chemical self-heating reaction
A non-combustible, self-heating technology, used in applications, smokers' products, heat exchange materials, etc., can solve the problems of few technical reports and short development time, and achieve good safety, high heating efficiency and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Design test of composition formula
[0066] 1. Orthogonal test verification
[0067] The present invention determines that the main components involved in the reaction are aluminum powder or iron powder, potassium permanganate, manganese dioxide, tartaric acid and potassium hydrogen tartrate, anhydrous magnesium sulfate, and the amount of activated carbon as the main influencing factors. This embodiment selects each factor Five levels, using a six-factor five-level L25 (56) orthogonal design scheme for experimental verification. In order to avoid repetition, this embodiment provides orthogonal test data of aluminum powder as an example for illustration.
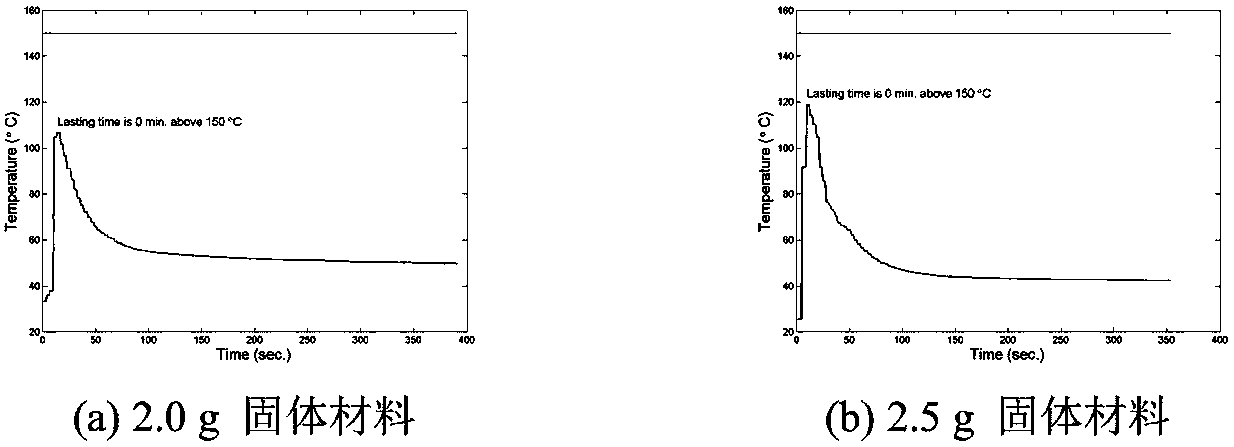
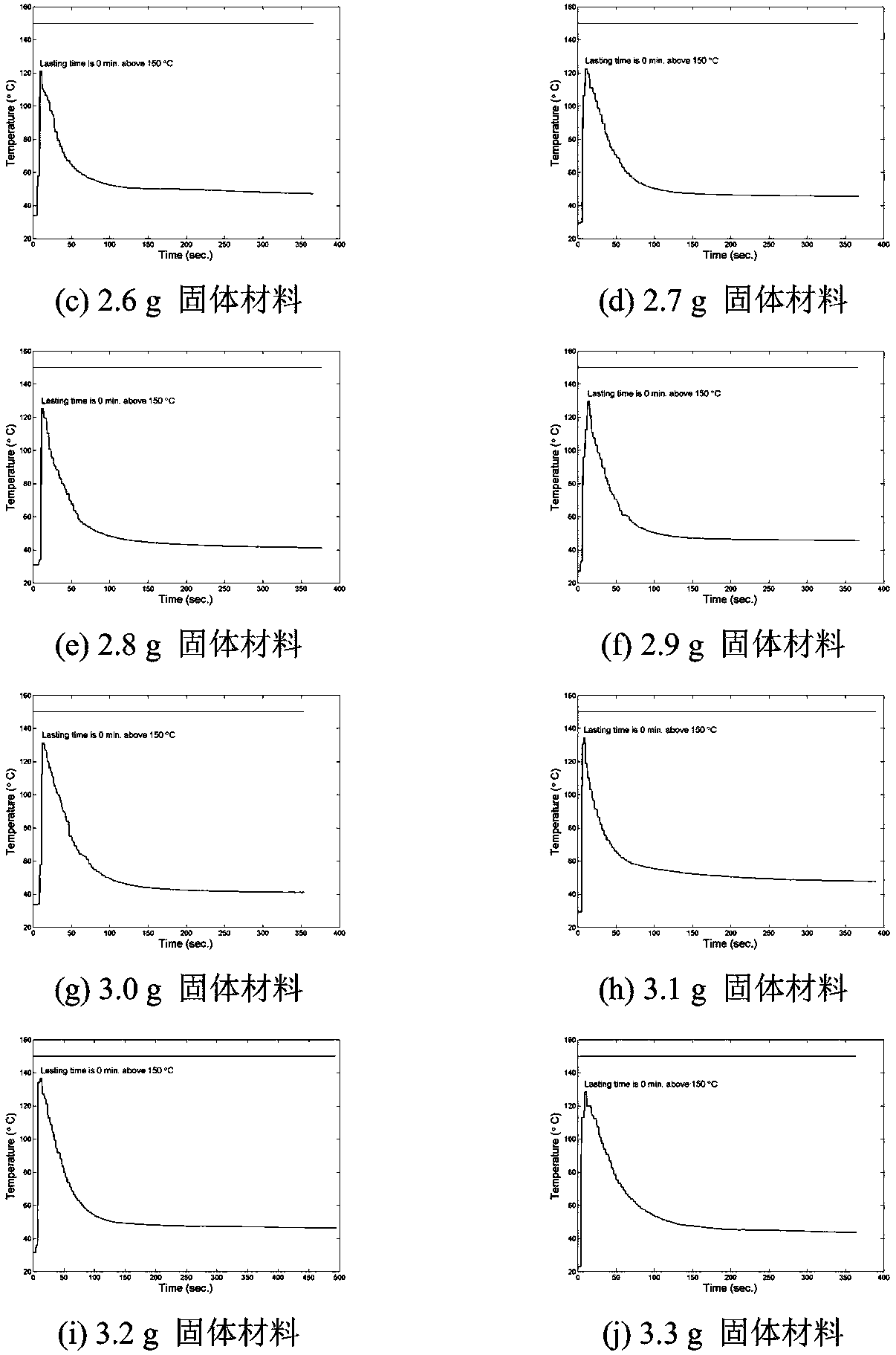
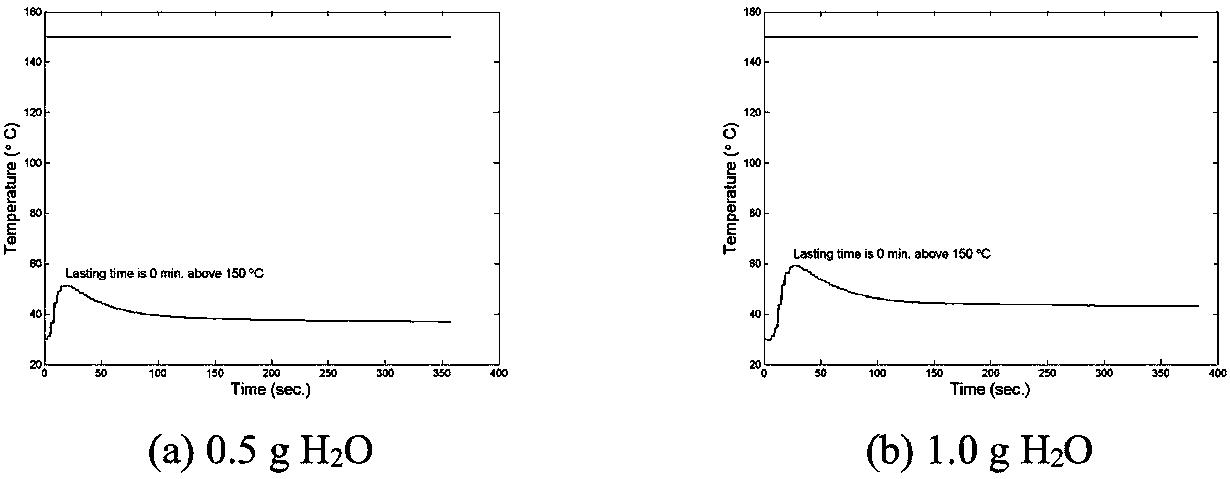
[0068] According to the orthogonal test design, determine the reasonable influencing factors and the level of dosage. Because aluminum powder or iron powder, potassium permanganate and acidic substances are necessary for the reaction, manganese dioxide, anhydrous magnesium sulfate, and activated carbon are not nece...
Embodiment 2
[0106] Example 2 Detection of overall response rate
[0107] According to the known reaction principle, combined with the addition amount of each reactant in the reaction, the theoretical value of manganese content in the sample can be calculated. The sampling position is divided into the whole reaction product, the inner and outer layers of the product, the upper layer of the product (the end closer to the copper tube cover), the middle layer and the lower layer (the end farther from the copper tube cover), and the weighed mixed solid heats up Material mass is m 0 . Knowing the proportion of potassium permanganate added, it can be known that the mass of potassium permanganate in the mixed solid material is m 1 , The mass of aluminum powder or iron powder added is m 2 , It can be calculated that the amounts of potassium permanganate and metal reactants are n 1 And n 2 , Where n 1 =m 2 / M KMnO4 , N 2 =m metal / M metal . An excess of potassium permanganate is added to the react...
Embodiment 3
[0128] Example 3 Application experiment
[0129] The materials were prepared and mixed according to the following mass ratio: iron powder 12.0%; potassium permanganate 61.1%; acidic substance tartaric acid 11.9%; activated carbon 2.5%; sodium chloride 12.5%.
[0130] The weighed solid material powder is added to the closed copper tube, and the water is added to the closed copper tube for reaction according to the mass ratio of mixture: water of 1.6:1. Use a thermometer to record the temperature changes during the entire reaction. Experimental results: The fever reaction can continue to exotherm above 250°C (up to 296°C) for more than 5 minutes, which is similar to the smoking time of a traditional cigarette.
[0131] The composition and water of this embodiment are placed in a copper tube (with a wall thickness of 0.5 mm) as a heating source for non-combustion cigarettes, which can ensure the heating effect, and the heating cavity is moderate, and the appearance is not much differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com