Recombinant human autoantigen Scl-70
A self-antigen, nucleic acid sequence technology, applied in the field of recombinant human self-antigen, can solve the problems of difficulty in extraction and purification of natural human antigens, limited development of ANA detection reagents, and difficulty in obtaining high-purity samples, and achieves strong reactivity, easy expression and The effect of purification and low cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: IPTG concentration is 0.2mM
[0033] The recombinant expression plasmid was introduced into Escherichia coli BL21 competent cells, and positive transformants were selected and inoculated in LB liquid medium containing kanamycin, and cultured overnight at 37°C on a shaking table. The next day, inoculate in 300ml of fresh same culture medium at a ratio of 1:100, culture on a shaker at 37°C until the A600 is 0.4-0.6, put the bacteria solution on ice for 30min, then add IPTG to a final concentration of 0.2mM, and overnight at 20°C induced expression.
Embodiment 2
[0034] Embodiment 2: IPTG concentration is 0.6mM
[0035] The recombinant expression plasmid was introduced into Escherichia coli BL21 competent cells, and positive transformants were selected and inoculated in LB liquid medium containing kanamycin, and cultured overnight at 37°C on a shaking table. The next day, inoculate 300ml of the same fresh culture medium at a ratio of 1:100, culture on a shaker at 37°C until the A600 is 0.4-0.6, put the bacteria in an ice bath for 30min, then add IPTG to a final concentration of 0.6mM, and overnight at 20°C induced expression.
Embodiment 3
[0036] Embodiment 3: IPTG concentration is 1.0mM
[0037] The recombinant expression plasmid was introduced into Escherichia coli BL21 competent cells, and positive transformants were selected and inoculated in LB liquid medium containing kanamycin, and cultured overnight at 37°C on a shaking table. The next day, inoculate 300ml of the same fresh culture medium at a ratio of 1:100, culture on a shaker at 37°C until the A600 is 0.4-0.6, put the bacteria in an ice bath for 30min, then add IPTG to a final concentration of 1.0mM, and overnight at 20°C induced expression.
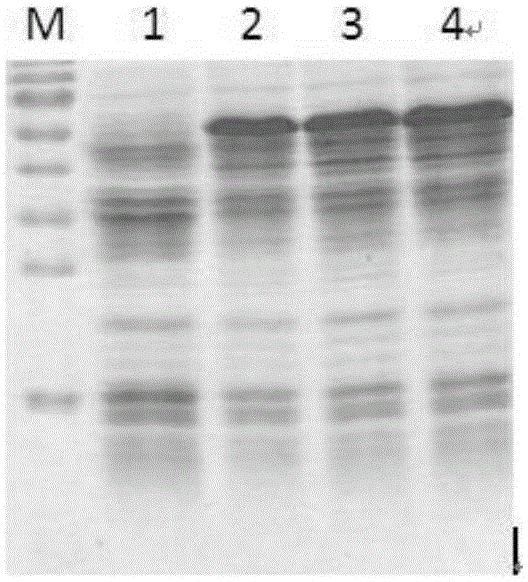
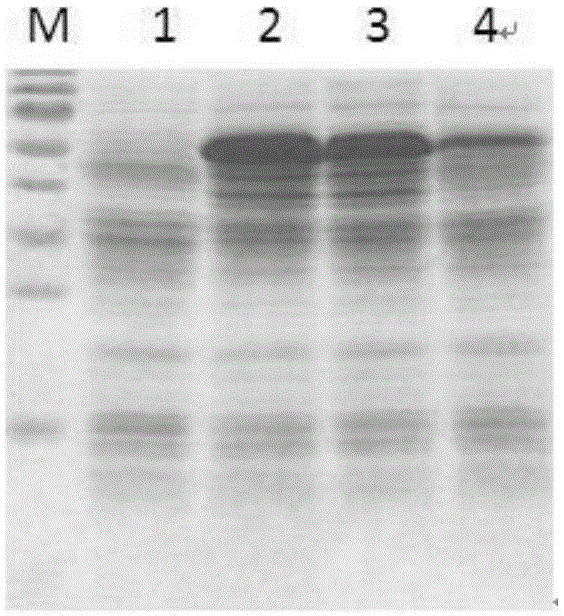
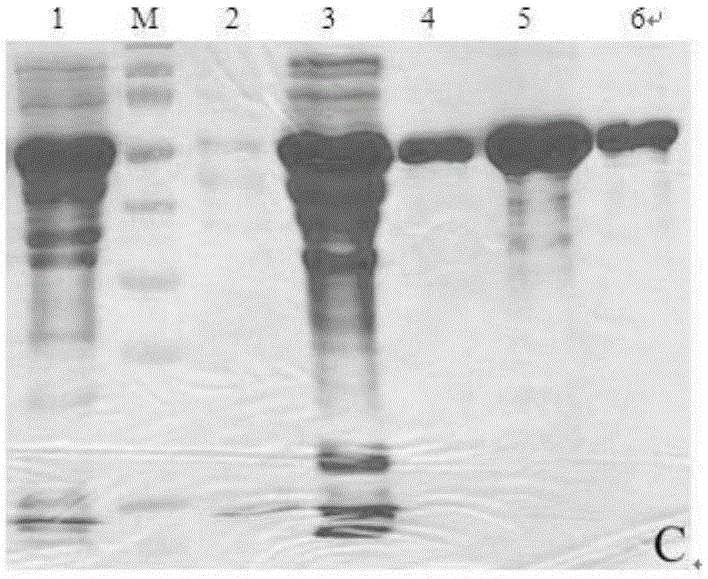
[0038] The expression results induced by different IPTG concentrations are as follows: figure 1 shown. figure 1 Middle, M: Protein Marker, the size from top to bottom is 130KD, 95KD, 72KD, 55KD, 43KD, 34KD, 26KD, 17KD, 10KD, 1: blank control without adding IPTG, 2: adding 0.2mMIPTG, 3: adding 0.6mMIPTG, 4: add 1.0mMIPTG
[0039] from figure 1 It can be seen that after adding IPTG, the recombinant human auto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com