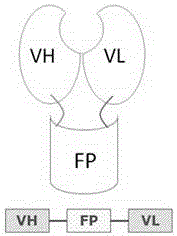
Structure for enhancing antibody drug stability
A technology for drugs and antibodies, applied in the field of genetic engineering, can solve the problems that antibody drugs are difficult to reach the target site, the drug efficacy is reduced, and the homodimer structure is easy to appear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Structural Design of Antibody Fusion Protein
[0021] The antibody fusion protein structure of the present invention is shown in figure 1 . VH and VL are the heavy chain variable region and light chain variable region of the antibody, respectively, and FP represents the recombinant protein. The three parts are connected by two peptide chains. Antibody fusion protein can be expressed by host cells through DNA recombinant technology.
Embodiment 2
[0022] Example 2 Antibody fusion protein drug
[0023] Fusion of the B5 antibody to human interleukin 2 (hIL2) to form the B5H-hIL2-B5L protein. The B5H-hIL2-B5L structure is that the heavy chain variable region of the protein B5 antibody is in the front, interleukin 2 is in the middle, and the light chain variable region of the B5 antibody is behind, and the three domains are connected by glycine-rich peptide chains . The amino acid sequence is shown in SEQ ID No.1.
[0024] The B5H-hIL2-B5L protein was obtained by secreting and expressing the protein from CHO (Chinese hamster ovary cells) cells and purifying it by cationic chromatography.
[0025] The purified B5H-hIL2-B5L protein was analyzed by SDS-PAGE denaturing electrophoresis and non-denaturing electrophoresis, and the molecular weight of the electrophoresis bands under both conditions was about 50kD. It suggested that B5H-hIL2-B5L was a monomer.
Embodiment 3
[0026] Example 3 Analysis of biological activity of antibody fusion protein
[0027] (1) IL2 biological activity of B5H-hIL2-B5L
[0028] B5H-IL2-B5L was added at 100 ng / ml to RPM1640 medium containing 10% fetal bovine serum to cultivate CTLL2 cells (mouse cytotoxic T lymphocytes), which can maintain the normal growth and continuous passage of CTLL2 cells. The hIL2 fused in the B5H-hIL2-B5L fusion protein has the biological activity of recombinant human IL2.
[0029] (2) Stability test of B5H-hIL2-B5L in plasma
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com