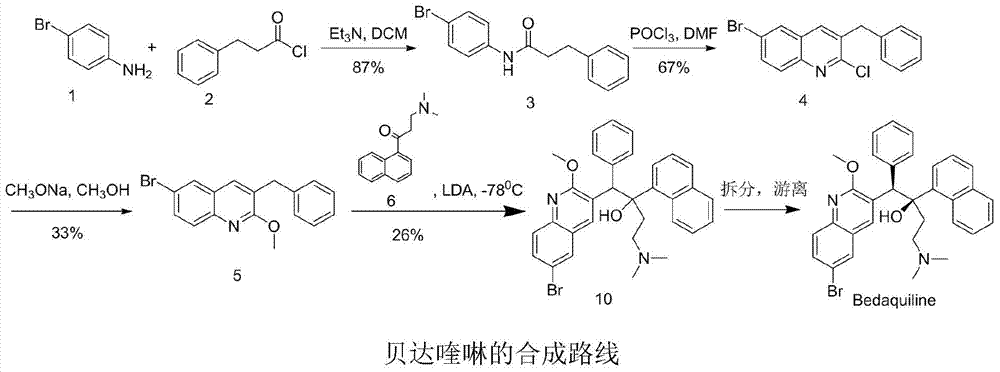
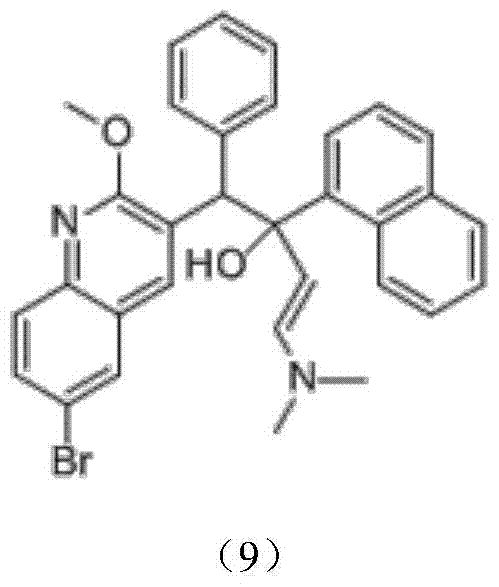
Intermediate for preparing bedaquiline and its preparation method and application
A bedaquiline and intermediate technology, which is applied in the field of preparation of intermediates for the treatment of multidrug-resistant tuberculosis drug bedaquiline, can solve the problems of low yield, incomplete conversion of raw materials, and poor purity of bedaquiline racemates. Advanced problems, to achieve the effect of high purity, great positive progress effect and practical application value, stable and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
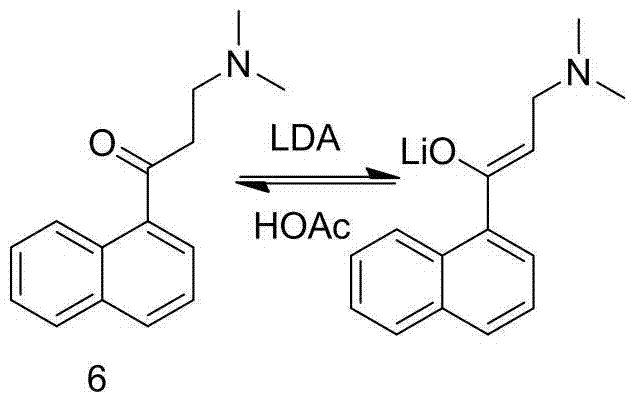
[0036] Preparation of 3-dimethylamino-2-(1-naphthyl)-prop-2-en-1-one (compound 8)
[0037] 1-Naphthyl ethyl ketone (170.0g, 1.0mol) was added to DMF-DMA (178.0g, 1.5mol) at room temperature, heated to 120°C, after 24h, cooled to room temperature, added 200ml of toluene to dilute, and then the solvent Evaporate to dryness under reduced pressure at 55°C, dilute the residue with 200ml of toluene, and evaporate to dryness under reduced pressure at 55°C to obtain 230.1g of a yellow oil, with a crude yield of 102% and a purity of 98.5% by HPLC, which can be directly used in the next reaction .
[0038] 1 H-NMR (CDCl 3 )δ: 2.93-3.14 (m, 6H); 5.73 (d, 1H, J = 12.4Hz); 7.38-7.49 (m, 2H); 7.82 (d, 1H, J = 12.4Hz); 7.78 (m, 1H ),8.14-3.18(m,2H),8.29(d,2H,J=8.4Hz),9.45(d,2H,J=8.8Hz).ESI-MS(m / z)=226.2[M+H] +
Embodiment 2
[0040] Preparation of 3-dimethylamino-2-(1-naphthyl)-prop-2-en-1-one (compound 8)
[0041] 1-Naphthylethanone (150.0g, 0.88mol) was added to DMF-DMA (57.5g, 1.5mol) at room temperature, heated to 90°C, after 24h, cooled to room temperature, added 200ml of toluene to dilute, and then the solvent Evaporate to dryness under reduced pressure at 55°C, add 200ml of toluene to the residue to dilute, and evaporate to dryness under reduced pressure at 55°C to obtain 202.5g of yellow oil, crude yield 102%, HPLC purity 98.8%. ESI-MS(m / z)=226.2[M+H] +
Embodiment 3
[0043]Preparation of 3-dimethylamino-2-(1-naphthyl)-prop-2-en-1-one (compound 8)
[0044] 1-Naphthyl ethyl ketone (250.0g, 1.47mol) was added to DMF-DMA (262.5g, 2.20mol) at room temperature, heated to 90°C, after 48h, cooled to room temperature, added 200ml of toluene to dilute, and then the solvent Evaporate to dryness under reduced pressure at 55°C, add 200ml of toluene to the residue to dilute, and evaporate to dryness under reduced pressure at 55°C to obtain 334.2g of yellow oil with a crude yield of 101% and an HPLC purity of 98.2%. ESI-MS(m / z)=226.2[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com