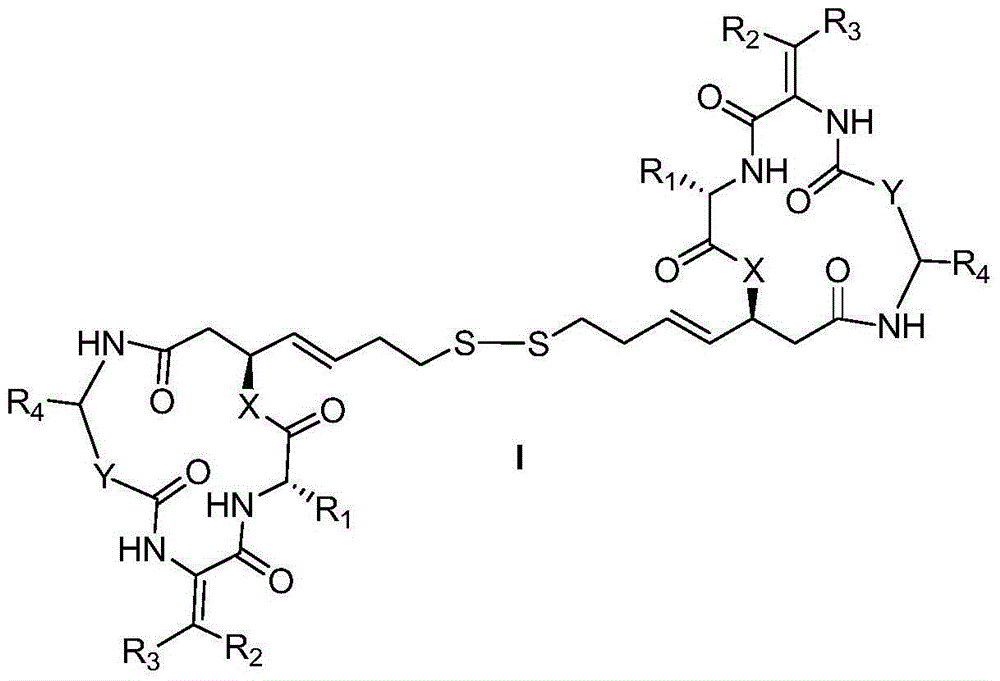
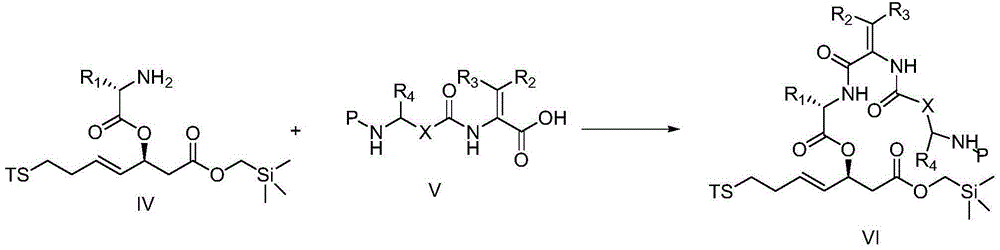
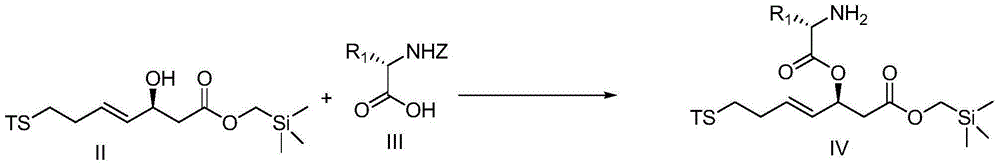Cyclic peptide compounds and application thereof
A technology of cyclic peptide compounds and solvates, which is applied in the field of medicinal chemistry and can solve problems such as apoptosis of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086]
[0087] Step 1. Synthesis of compound b
[0088]
[0089] Fmoc-L-Ala (3.11g, 10.0mmol), EDCI (1.91g, 10.0mmol) and DMAP (50mg, 0.406mmol) were dissolved in anhydrous dichloromethane (38mL), and DIPEA (1.6mL , 10.0mmol) and compound a (3.18g, 6.15mmol), stirred at room temperature for 12h, diluted with dichloromethane, the reaction solution was washed with sodium bicarbonate solution (70mLx3), the organic phase was washed with saturated brine, anhydrous sodium sulfate After drying and concentration, the residue was subjected to silica gel column chromatography to obtain compound 9 (4.49 g, 90%) as a pale yellow solid. [α] 20 D :-15.0 (c1.10, CHCl 3 ). 1 HNMR (400MHz, CDCl 3 ): δ7.73(d, J=7.6Hz, 2H), 7.57(m, 2H), 7.38(dd, J=7.4Hz, 2H), 7.30(dd, J=7.4Hz, 2H), 7.37-7.13 (m,15H),5.82and5.73(m,1H),5.65(dd,J=13.6,7.2Hz,1H),5.50(dd,J=15.2,7.2Hz,1H),5.31(d,J= 8.8Hz, 1H), 4.36(t, J=6.8Hz, 2H), 4.25(dd, J=9.2, 4.6Hz, 1H), 4.21(t, J=7.2, 1H), 4.12(t, J=8.4 Hz, 2H), 2...
Embodiment 2
[0102] Embodiment 2, the synthesis of compound 1-2
[0103] The synthesis method is as in Example 1.
[0104] [α] 20 D =8.6(c0.20, CHCl 3 ). 1 HNMR (400MHz, CDCl 3 )δ8.55(s,1H),8.10(s,1H),6.92(q,J=7.0Hz,2H),6.51(d,J=10.0Hz,1H),5.82–5.61(m,2H), 5.53(dd, J=15.6,6.8Hz,1H),5.20(dd,J=17.4,8.0Hz,1H),4.73(dd,J=10.2,3.8Hz,1H),4.32(dd,J=17.7, 4.1Hz,1H),2.88–2.76(m,2H),2.73–2.60(m,3H),2.47–2.33(m,2H),2.30(d,J=6.7Hz,3H)ppm. 13 CNMR (125MHz, CDCl 3 )δ169.5, 168.8, 167.2, 163.2, 158.1, 148.0, 134.2, 132.0, 128.5, 127.0, 124.2, 71.8, 40.6, 40.2, 37.2, 31.3, 31.0ppm. HRMS (ESI) Calcdm / zforC 36 h 43 N 8 o 10 S 4 [(M+H) + ]875.1985, found 875.1986.
Embodiment 3
[0105] Embodiment 3, the synthesis of compound 1-3
[0106] The synthesis method is as in Example 1.
[0107] [α] 20 D =11.6(c0.20, CHCl 3 ). 1 HNMR (400MHz, CDCl 3 )δ8.57(s,1H),8.13(s,1H),6.94(q,J=7.1Hz,2H),6.51(d,J=10.2Hz,1H),5.85–5.65(m,2H), 5.54(dd, J=15.6,6.8Hz,1H),5.20(dd,J=17.6,8.2Hz,1H),4.75(dd,J=10.2,3.8Hz,1H),4.33(dd,J=17.7, 4.1Hz, 1H), 2.90–2.75(m, 1H), 2.70–2.60(m, 3H), 2.48–2.35(m, 2H), 2.29(m, 1H), 1.82(d, J=7.1Hz, 3H ),0.80(t,J=6.8Hz,3H)ppm. 13 CNMR (125MHz, CDCl 3 )δ169.7, 169.1, 167.3, 163.5, 158.3, 148.0, 134.4, 132.1, 128.6, 127.0, 124.2, 71.8, 57.0, 40.7, 40.2, 37.3, 31.5, 31.2, 19.0, 16.3, ppm.HRMS( / ESI)Calcdm 40 h 51 N 8 o 10 S 4 [(M+H) + ]931.2611,found931.2612.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com