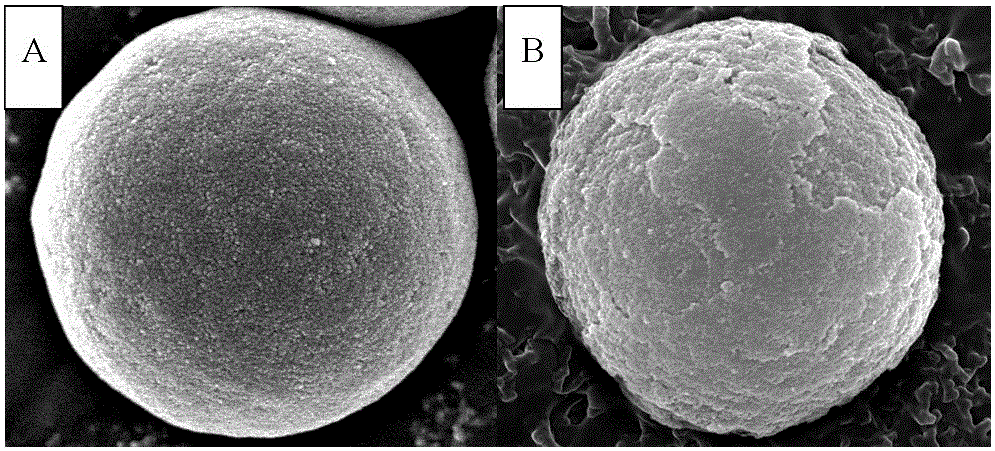
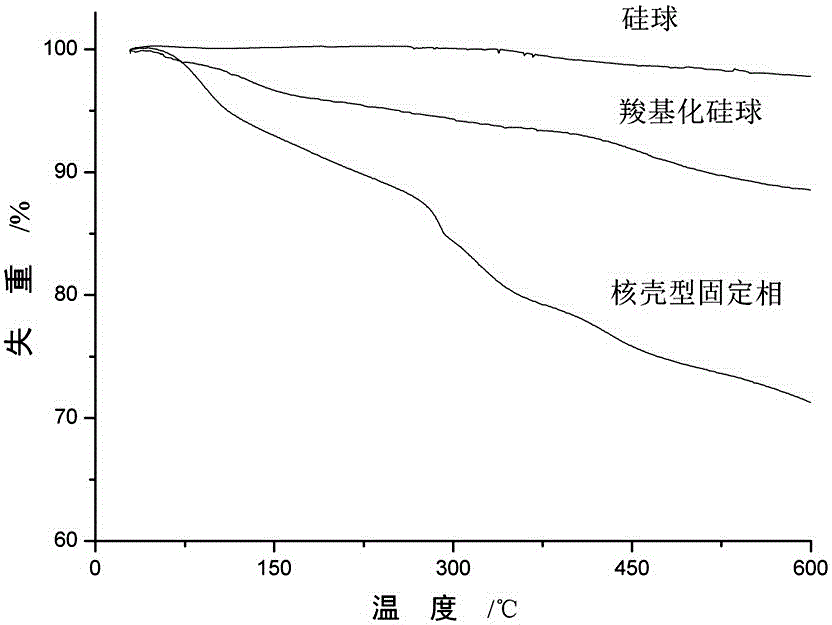
Core-shell type hydrophilic chromatographic stationary phase with metal organic framework material as shell, preparation method and application thereof
A metal-organic framework and hydrophilic chromatography technology, which is applied in the field of core-shell type hydrophilic chromatography stationary phase and its preparation, can solve the problems of narrow application range and low column efficiency, and achieves easy popularization and application, uniform distribution and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Silica gel pretreatment: Add glutaric anhydride and silicon spheres bonded with amino groups to N,N-dimethylformamide, the mass ratio of the two is 2:1, react at 30°C for 24h, after the reaction, centrifuge The solvent was removed, and the obtained silicon spheres were washed three times with absolute ethanol to remove unreacted glutaric anhydride to obtain carboxyl-functionalized silicon spheres.
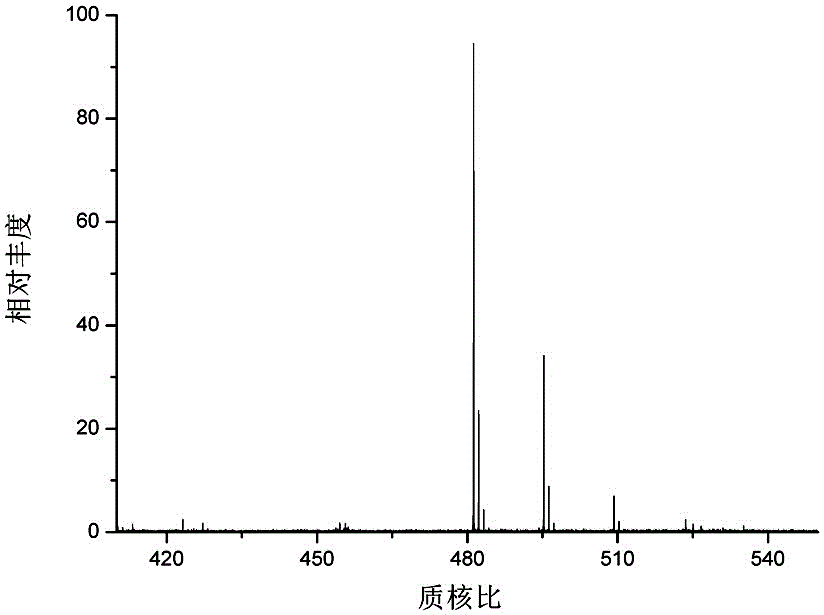
[0028] Synthesis of 1,3-bis(4-carboxybutyl)imidazole bromide: under nitrogen protection, use methyl 4-bromobutyrate and N-trimethylsilimidazole as raw materials, and control the molar ratio of the two 2:1, reacted at 60°C for 24h, after the reaction was completed, washed 3 times with anhydrous ether to remove unreacted methyl 4-bromobutyrate to obtain ester-modified imidazole; add 37% concentrated hydrochloric acid to control the two The molar ratio is 1:2.5, reflux reaction at 100°C for 2 hours, after the reaction is completed, the solvent is removed by rotary evaporation, ...
Embodiment 2
[0034] Silica gel pretreatment: Add glutaric anhydride and silicon spheres bonded with amino groups to N,N-dimethylformamide, the mass ratio of the two is 3:1, react at 30°C for 24h, after the reaction, centrifuge The solvent was removed, and the obtained silicon spheres were washed three times with absolute ethanol to remove unreacted glutaric anhydride to obtain carboxyl-functionalized silicon spheres.
[0035] Synthesis of 1,3-bis(4-carboxybutyl)imidazole bromide: under nitrogen protection, use methyl 4-bromobutyrate and N-trimethylsilimidazole as raw materials, and control the molar ratio of the two 3:1, reacted at 60°C for 24h, after the reaction was completed, washed 3 times with anhydrous ether to remove unreacted methyl 4-bromobutyrate to obtain ester-modified imidazole; add 37% concentrated hydrochloric acid to control the two The molar ratio is 1:2.5, reflux reaction at 100°C for 2 hours, after the reaction is completed, the solvent is removed by rotary evaporation, ...
Embodiment 3
[0038] Silica gel pretreatment: Add glutaric anhydride and silicon spheres bonded with amino groups to N,N-dimethylformamide, the mass ratio of the two is 2:1, react at 30°C for 24h, after the reaction, centrifuge The solvent was removed, and the obtained silicon spheres were washed three times with absolute ethanol to remove unreacted glutaric anhydride to obtain carboxyl-functionalized silicon spheres.
[0039] Synthesis of chlorinated 1,3-bis(4-carboxybutyl)imidazole: under nitrogen protection, use methyl 4-chlorobutyrate and N-trimethylsilimidazole as raw materials, and control the molar ratio of the two 1:1, reacted at 60°C for 24h, after the reaction was completed, washed 3 times with anhydrous ether to remove unreacted methyl 4-chlorobutyrate to obtain ester-modified imidazole; add 37% concentrated hydrochloric acid to control two The molar ratio is 1:2.5, reflux reaction at 100°C for 2 hours, after the reaction is completed, the solvent is removed by rotary evaporation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com