Duck parvovirus strain and live vaccine thereof
A duck parvovirus and live vaccine technology, applied in the field of animal virology, can solve the problems of low potency and inability to use vaccines for prevention and control, and achieve the effects of ensuring healthy development, good immunogenicity and safety, and reducing breeding risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Isolation and identification of embodiment 1GT2015 virus
[0033] In April 2015, the affected ducks collected from the Gaotang area of Shandong were clinically manifested as growth retardation and atrophy of the upper and lower beaks. Isolate and identify the isolated strains by PCR, EID50 toxicity test, animal regression test, etc. The results showed that the target fragment was successfully amplified; the virus virulence was 10 6.7 EID50 / 0.2mL; The inoculated ducks in the animal infection test had obvious clinical symptoms and pathological changes, which were consistent with the clinical diseased ducks. Thus, it was determined that the isolated strain was duck parvovirus, and it was named GT2015 strain, and the inventor carried out a biological deposit on it, and the deposit number was CCTCC No: V201527.
Embodiment 2
[0034] Obtaining of embodiment 2GT2015v virus and identification of its biological characteristics
[0035] Acquisition of GT2015v virus
[0036] The duck parvovirus GT2015 strain was inoculated into 9-day-old duck embryos for 40 generations, and the attenuated strain was cloned and purified, which was named GT2015v strain. Virus titer was measured as 10 6.6 EID50 / 0.2mL. It shows that the attenuated strain has good reproduction ability in duck embryo. The inventor has carried out a biological deposit on it, and the depository unit is the China Center for Type Culture Collection, and the biological deposit number is CCTCCNo: V201528.
[0037] Biological characteristics of duck parvovirus GT2015v
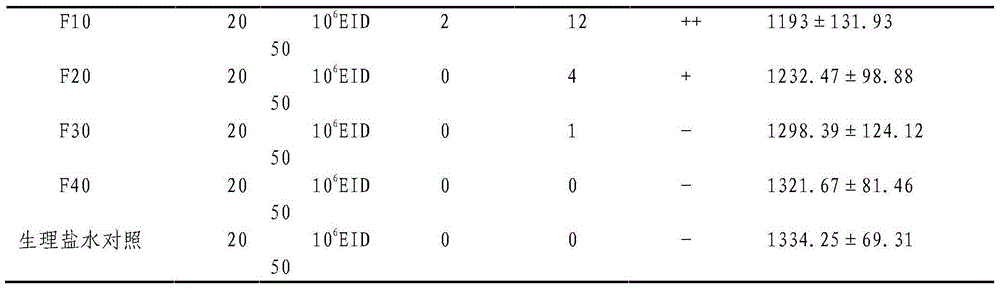
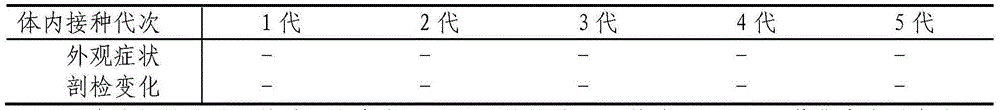
[0038] 1 Safety test: 100 1-day-old ducklings were divided into 5 groups on average, and 10 6 F10, F20, F30 and F40 generation strains of EID50, and set a blank control group. Symptoms were observed and weighed for 25 consecutive days. Every five days, two animals in each group...
Embodiment 3
[0046] Embodiment 3 Vaccine finished product preparation
[0047] Nine-day-old duck embryos were inoculated with the attenuated GT2015v strain of duck parvovirus, incubated at 37°C for 5 days, and the virus allantoic fluid was harvested, added with lyoprotectant, and vacuum freeze-dried to prepare duck parvovirus live vaccine.
[0048] The preparation method of the duck parvovirus live vaccine provided by the invention comprises inoculating duck embryos with duck parvovirus attenuated GT2015v strain, harvesting virus cultures, adding a stabilizing protective agent, and drying in vacuum.
[0049] Propagation of vaccine strains: Well-developed 9-day-old healthy duck embryos were selected for virus propagation, and the virus solution of duck parvovirus-induced attenuated GT2015v strain was inoculated at 0.2mL / embryo, cultured at 37°C for 5 days, and the virus allantoic fluid was collected and stored at -20°C save.
[0050] Inspection of semi-finished products:
[0051] Sterility ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com