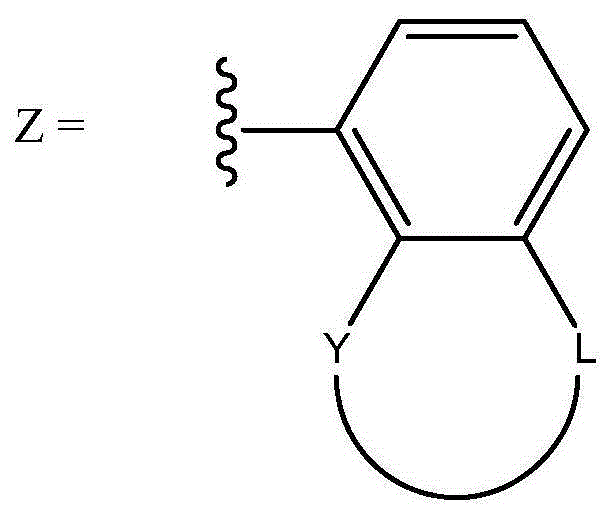
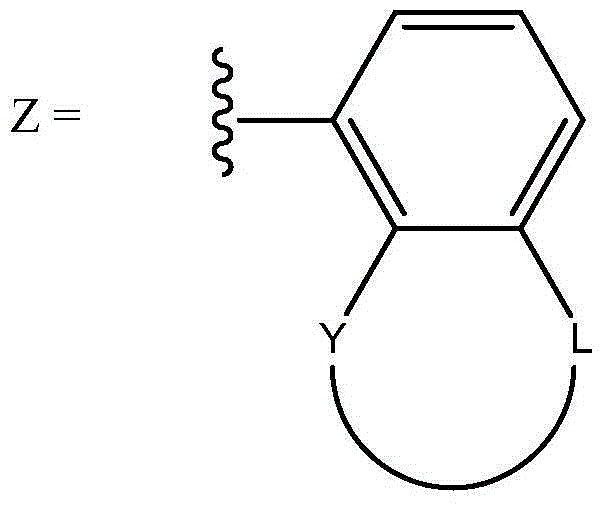
Oligomerisation of ethylene to mixtures of 1-hexene and 1-octene
A technology of mixture and hexene, applied in the field of oligomerization of ethylene oligomerization into a mixture of 1-hexene and 1-octene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0127] In the preparation of the catalyst system used in the present invention, the optimum amount of compound to be used for activation can be easily determined by simple tests, for example, by the preparation of small test samples, which can be used for oligomerization A small amount of ethylene and thus determine the activity of the resulting catalyst. It has generally been found that for aluminum alkyls and aluminoxane-type activators or co-activators, a suitable use level is from 0.5 to 2000 moles of aluminum per mole of chromium.
[0128] Examples of suitable organoboron activator compounds are boronoxane, NaBH 4 , trimethylboron, triethylboron, triphenylboron, dimethylphenylammonium tetrakis (phenyl) borate, trityl tetrakis (phenyl) borate, tetrakis (pentafluorophenyl) boric acid Dimethylphenylammonium, Trityltetrakis(pentafluorophenyl)borate, Tris(pentafluorophenyl)boron, Sodium tetrakis[(bis-3,5-trifluoromethyl)phenyl]borate , Dimethylphenylammonium tetrakis[(bis-3,...
Embodiment 1
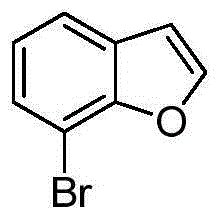
[0301] Example 1 utilizes (1-benzofuran-7-yl) at 60°C and 45 bar 2 PN(n-hexyl)P(phenyl) 2 the B ene tetramer
[0302] A 600 ml stainless steel reactor was heated to 120 °C under vacuum for 30 min with N 2 Backfill, then cool to 60°C. The reactor was filled with 2,2,4-trimethylpentane (TMP) (100 ml) and heated to 60°C. Separately, MMAO-3A (2.4mmolAl) was added to Cr(acac) 3 (2.5 μmol) and (1-benzofuran-7-yl) 2 PN(n-hexyl)P(phenyl) 2 (2.5 μmol) in cyclohexane (5 ml). Then, this mixture was transferred to the reactor. The reactor was pressurized with ethylene (45 bar) and stirred with a gas-entraining stirrer (1300 r.p.m.). The temperature in the reactor was raised to 62-65°C, at which point the reactor was cooled with an internal cooling coil to maintain a constant temperature of 60°C throughout the run. The reaction pressure was kept constant throughout the run by supplying ethylene on demand and the consumption of ethylene was monitored via a flow meter. ...
Embodiment 2
[0303] Example 2 using (1-benzofuran-7-yl)(phenyl)PN(n-hexyl)P(phenyl) at 60°C and 45 bar 2 ethylene tetramer
[0304] According to the steps of Example 1, the difference is: use 1.0mmolAl as MMAO-3A, 1.0μmolCr(acac) 3 and the ligand (1-benzofuran-7-yl)(phenyl)PN(n-hexyl)P(phenyl) 2 (1.0 μmol), and the reaction was terminated after 17 minutes and 150 g of ethylene input. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com