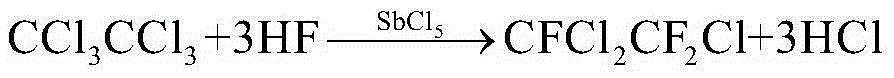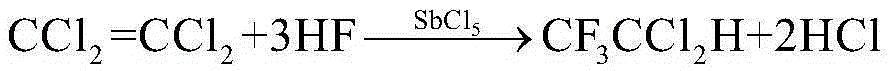Method for combined production of 1,1,2-trifluorotrichloroethane and 1,1,1-trifluorodichloroethane
A technology of trifluorodichloroethane and trifluorotrichloroethane, applied in the field of co-production of 1,1,2-trifluorotrichloroethane and 1,1,1-trifluorodichloroethane, It can solve the problems of low equipment production capacity, complex operation, and many by-products, and achieve the effects of easy control of reaction temperature, rich source of raw materials, and easy separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]In a 1L reaction autoclave, add 250g (1.05mol) of hexachloroethane, 250g (1.51mol) of tetrachloroethylene, 100g (0.33mol) of catalyst antimony pentachloride, and 400g (20mol) of hydrofluoric acid at one time, and start Stir, heat up to 90°C, and react for 8 hours. During the reaction, the pressure should be released regularly to keep the pressure at 1.5Mpa. When the pressure indication remains unchanged, stop heating and cool down. When the temperature drops below 25°C, discharge the material, wash with water, wash with alkali, rectify and purify 1,1,2-trifluorotrichloroethane and 1,1,1-trifluorodichloroethane respectively. 168.12 g of 1,1,2-trifluorotrichloroethane was obtained, with a yield of 85.2%, and 205.54 g of 1,1,1-trifluorodichloroethane, with a yield of 89.2%.
Embodiment 2
[0037] In a 1L reaction autoclave, add 250g (1.05mol) of hexachloroethane, 250g (1.51mol) of tetrachloroethylene, 100g (0.46mol) of catalyst antimony pentafluoride, and 400g (20mol) of hydrofluoric acid at one time. Stir, heat up to 120°C, and react for 7 hours. During the reaction, the pressure should be released regularly to keep the pressure at 1.5Mpa. When the pressure indication remains unchanged, stop heating and cool down. When the temperature drops below 25°C, discharge the material, wash with water, wash with alkali, rectify and purify 1,1,2-trifluorotrichloroethane and 1,1,1-trifluorodichloroethane respectively. 178.65 g of 1,1,2-trifluorotrichloroethane was obtained, with a yield of 90.33%, and 214.54 g of 1,1,1-trifluorodichloroethane, with a yield of 94.2%.
Embodiment 3
[0039] In a 1L reaction autoclave, add 300g (1.27mol) of hexachloroethane, 200g (1.2mol) of tetrachlorethylene, 100g (0.33mol) of catalyst antimony pentachloride, and 400g (20mol) of hydrofluoric acid at one time, and start Stir, heat up to 120°C, and react for 8 hours. During the reaction, the pressure should be released regularly to keep the pressure at 1.5Mpa. When the pressure indication remains unchanged, stop heating and cool down. When the temperature drops below 25°C, discharge the material, wash with water, wash with alkali, rectify and purify 1,1,2-trifluorotrichloroethane and 1,1,1-trifluorodichloroethane respectively. 222.15 g of 1,1,2-trifluorotrichloroethane was obtained, with a yield of 93.6%, and 162.77 g of 1,1,1-trifluorodichloroethane, with a yield of 88.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com