A method for co-producing methyl benzyl alcohol, methyl benzaldehyde and methyl benzoic acid
A technology of methyl benzaldehyde and methyl benzoic acid, applied in chemical instruments and methods, carboxylate preparation, carbon-based compound preparation, etc., can solve the problems of many by-products, difficult separation, long reaction time, etc., and achieve activity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
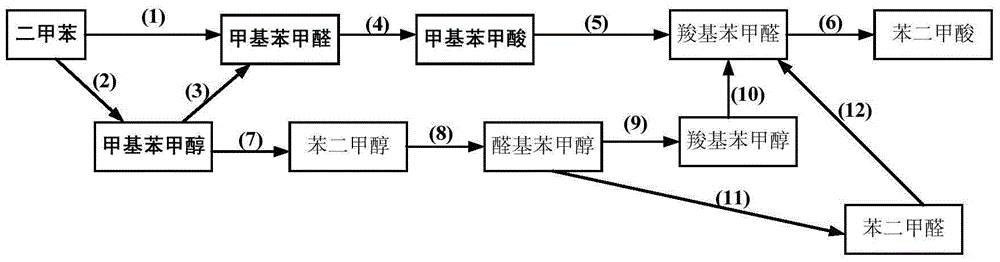
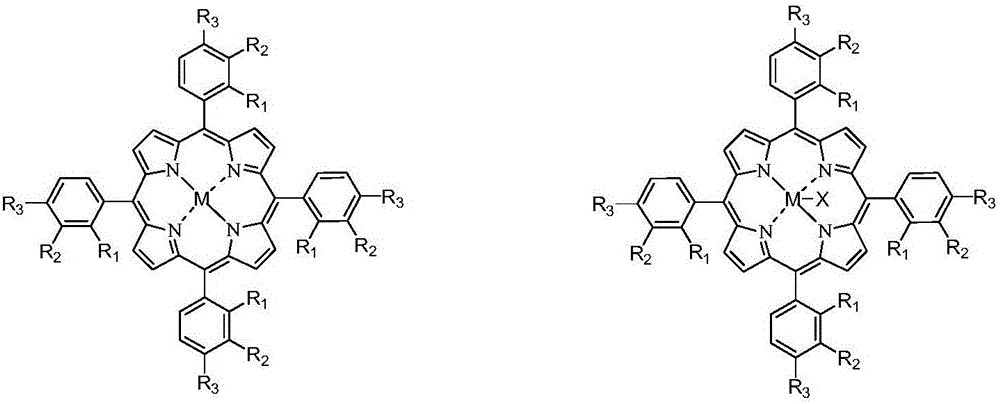
[0058] Add 550g o-xylene into a 1L batch oxidation reactor, and add 600ppm (concentration in o-xylene) of HfO 2 , N-hydroxy sulfonyl benzamide, metal phthalocyanine with general formula (IV) structure (R 1 =H, R 2 =F, M=Fe) and metalloporphyrin (R) with the structure of general formula (I) 1 =R 3 =OH, R 2 =H, M=Ru) is the catalyst, 27.5g of o-carboxybenzaldehyde and o-phthalaldehyde is the mixture of co-oxidant, and then 1.2MPa of air is continuously introduced, and the tail oxygen concentration is controlled by controlling the amount of air. Not more than 5%, keep the pressure in the reactor at 0.8 MPa and the temperature at 145° C. to continuously stir the reactants, stop the reaction after 1.6 hours, quickly cool the reactants and take samples for analysis. The analysis result of liquid chromatography showed that the conversion rate of o-xylene was 8.8%, the total selectivity of alkyd in the final oxidation product was 98.5%, and the total selectivity of aldol was 88.2%. The ...
Embodiment 2
[0060] In a 1L batch oxidation reactor, add 550g of o-xylene, and add 660ppm (concentration in o-xylene) of N-hydroxy-4-nitrophthalimide, with the general formula ( IV) Metal phthalocyanine of structure (R 1 =NH 2 , R 2 =H, M=Cu) and the metalloporphyrin (R 1 =R 3 =H, R 2 =CH 3 , M 1 =M 2 =Cr) is a catalyst, 16.6g o-methylbenzaldehyde is a co-oxidant, and then 2.0MPa of air is continuously introduced. By controlling the amount of air introduced, the tail oxygen concentration is controlled not to exceed 5%, and the pressure in the reactor is maintained at The reactant was continuously stirred under the conditions of 1.6 MPa and the temperature of 130° C., the reaction was stopped after 1.4 hours, and the reactant was quickly cooled and sampled for analysis. The analysis result of liquid chromatography showed that the conversion rate of o-xylene was 6.8%, the total selectivity of aldol in the final oxidation product was 98.8%, and the total selectivity of aldol was 90.9%. The sel...
Embodiment 3
[0062] In a 1L batch oxidation reactor, add 550g of p-xylene, and add 800ppm (concentration in p-xylene) of cobalt naphthenate, metal phthalocyanine with general formula (IV) structure (R 1 =CH 3 CH 2 , R 2 =H, M = Mn), metal porphyrin (R 1 =R 2 =H, R 3 =CH 3 , M=Cu) is a catalyst, 99.0g of p-carboxybenzyl alcohol, p-methylbenzaldehyde and p-methylbenzyl alcohol is a mixture of co-oxidant, and then 1.0MPa of air is continuously introduced by controlling the air inflow The volume control tail oxygen concentration does not exceed 5%, and the reactant is continuously stirred under the conditions of maintaining the pressure in the reactor at 0.5 MPa and the temperature at 130°C. The reaction is stopped after 5.0 hours, and the reactant is quickly cooled and sampled for analysis. The analysis result of liquid chromatography showed that the conversion rate of p-xylene was 11.9%, the total selectivity of aldol in the final oxidation product was 98.0%, and the total selectivity of aldol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com