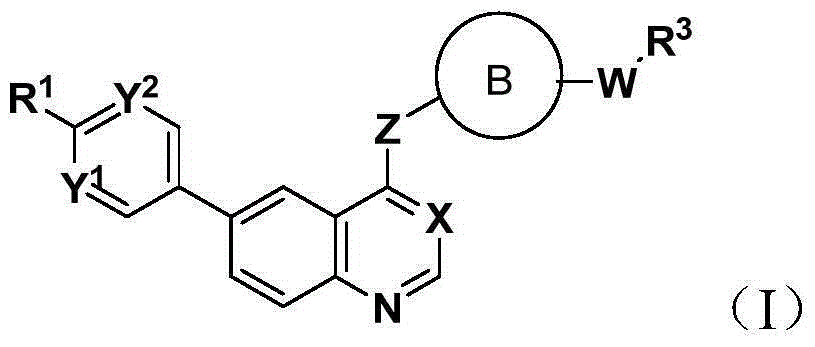
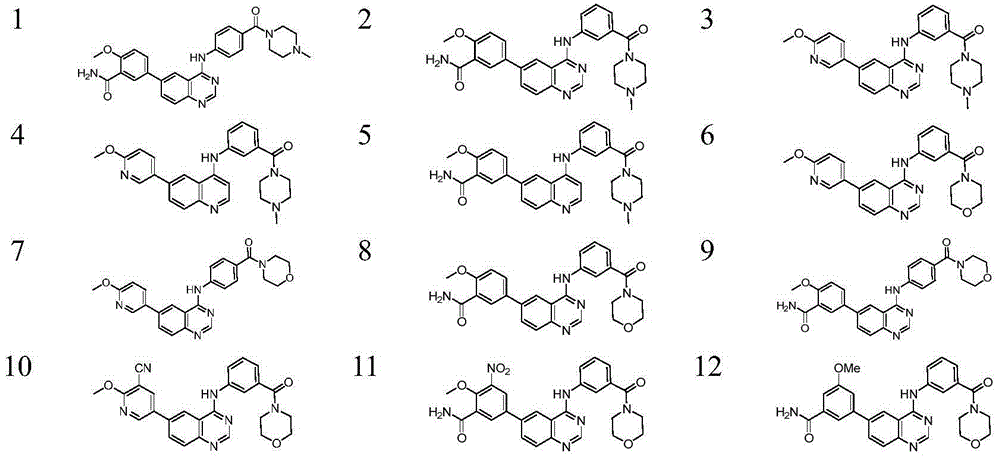
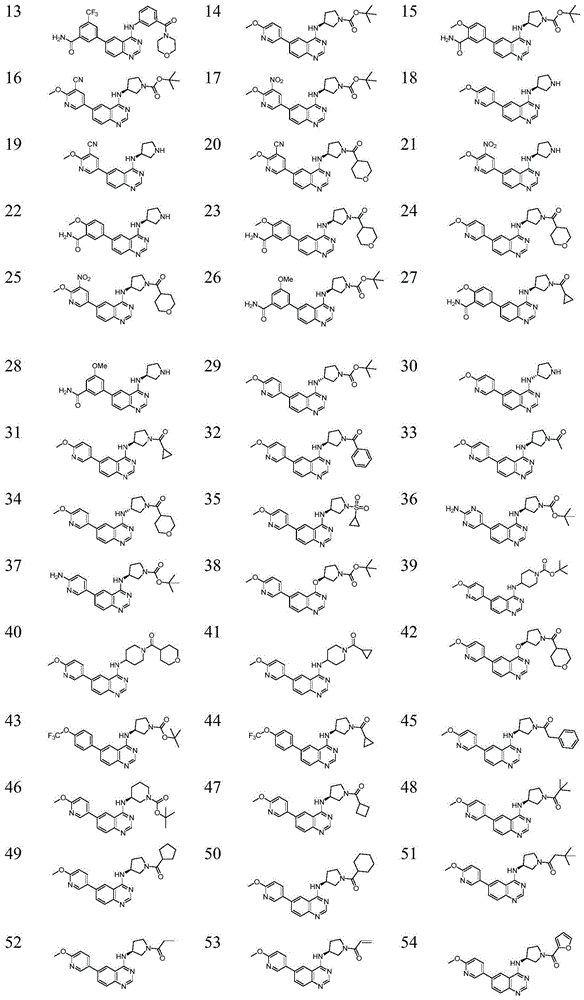
6-aryl substituted quinoline compounds and applications thereof
A compound and quinoline technology, applied in the field of 6-aryl substituted quinazolines and quinoline compounds, can solve the problems of underdeveloped value, narrow indications, and high toxicity of idelalisib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093]
[0094] 4-(4-(4-methylpiperazine-1-carbonyl)phenyl)amino-6-bromoquinazoline (intermediate 1-c) weighs 4-chloro-6-bromoquinazoline (1- a, 0.16g, 0.58mmol) was placed in isopropanol (5mL), 4-(4-methylpiperazine-1-carbonyl)aniline (0.14g, 0.64mmol) was added, and the temperature was raised to reflux for 5h under nitrogen protection, Cool to room temperature and filter to obtain white solid 1-c (0.18g, 73%). MS(ESI)m / z:[M+H] + =426.1,428.1.
[0095] 2-methoxy-5-(4-((4-(4-methylpiperazine-1-carbonyl)phenyl)amino)quinazolin-6-yl)benzamide (compound 1) weighed 2 -Methoxy-5-bromobenzamide (50mg, 0.22mmol) was placed in a 50mL bottle, dioxane (5mL) was added, and biboronic acid pinacol ester (60mg, 0.24mmol), potassium acetate ( 54mg, 0.55mmol), Pd(dppf)Cl 2 (12mg, 0.0165mmol), reflux reaction in a nitrogen atmosphere for 4h, the filtrate was concentrated to dryness, and the crude product 1-e was obtained; then intermediate 1-c (75mg, 0.22mmol), sodium carbonate (58mg, 0...
Embodiment 2
[0097]
[0098]4-(3-(4-methylpiperazine-1-carbonyl)phenyl)amino-6-bromoquinazoline (intermediate 2-c) can be prepared by a method similar to compound 1-c to obtain compound 2 -c (0.43g, 62%). M.p.256-258℃, MS(ESI)m / z:[M+H] + =426.1,428.1.
[0099] 2-methoxy-5-(4-((3-(4-methylpiperazine-1-carbonyl)phenyl)amino)quinazolin-6-yl)benzamide (compound 2) was prepared using Compound 2, a white solid (168 mg, 68%), could be prepared in a similar manner to 1. M.p.205-207℃, MS (ESI) m / z: [M+H] + =497.2. 1 H-NMR (400M, DMSO-d 6 )δ10.16(s,1H),8.85(s,1H),8.62(d,1H),8.30(d,1H),8.19(s,1H),7.99(d,3H),7.89(d,1H ),7.77(s,1H),7.68(s,1H),7.51(d,1H),7.36-7.29(m,1H),7.19(s,1H),3.98(s,3H),3.80-3.53( m,4H),2.75-2.45(m,4H),2.38(s,3H)ppm.
Embodiment 3
[0101]
[0102] 4-(3-(4-methylpiperazine-1-carbonyl) phenyl) amino-6-(2-methoxypyridin-5-yl) quinazoline (compound 3) preparation compound 1 similar method Compound 3 (50 mg, 11%) could be obtained. M.p.252-256℃, MS(ESI)m / z:[M+H] + =455.2. 1 H-NMR (400M, DMSO-d 6 )δ10.14(s,1H),8.95(s,1H),8.76(s,1H),8.68(s,1H),8.40-8.26(m,1H),8.22(d,1H),8.08-7.94 (m,2H),7.83(d,1H),7.49(t,1H),7.16(d,1H),7.02(d,1H),3.94(s,3H),3.54-3.34(m,4H), 2.48-2.28(m,4H),2.23(s,3H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com