Expression vector
A technology for expression vectors and variants, applied in the field of expression vectors, can solve the problems of death, inability to achieve long-term expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1. Host cells
[0076] Tn-5 cell line: BTI-TN-5B1-4 cell line (also known as HighFive or Tn-5) from Trichoplusiani, adapted to suspension culture and protein-free medium.
[0077] Proliferation of Tn-5 cells:
[0078] Tn-5 cells were obtained from commercial sources (LifeTechnologies, Basel, Switzerland) and cultured in suspension in SF900IISFM medium (LifeTechnologies). Cells were cultured in TubeSpin Bioreactor 50 (TS50) or in TubeSpin Bioreactor 600 (TS600) (TPP, Trasadingen, Switzerland) in volumes of 10 mL or 300 mL of the respective medium. Cells were passaged 3 times a week at a density of 1-5x105 cells / mL. All cultures were maintained at a temperature of 28° C. in an ISF1-X shaking incubator (Kühner AG, Birsfelden, Switzerland) with a shaking speed of 180 rpm and a shaking diameter of 5 cm. Cell density and viability were determined by trypan blue exclusion using a Neubauer hemocytometer.
Embodiment 2
[0079] Example 2. Plasmid preparation
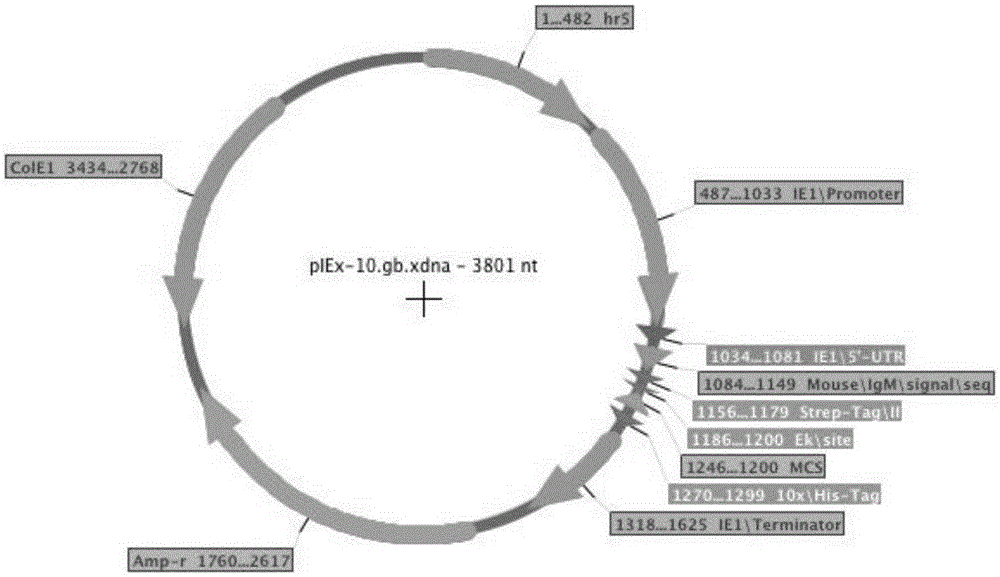
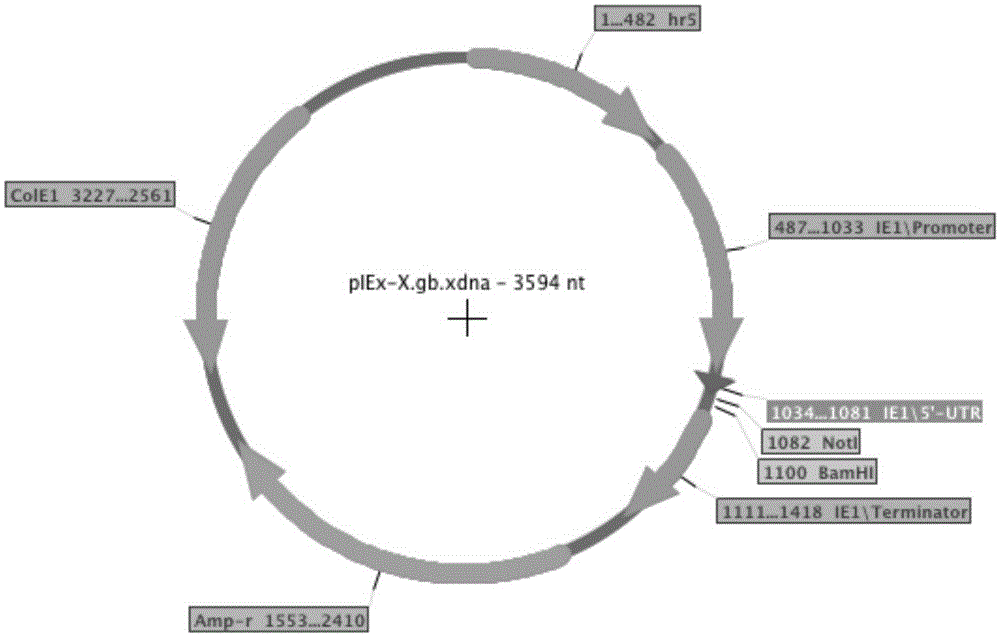
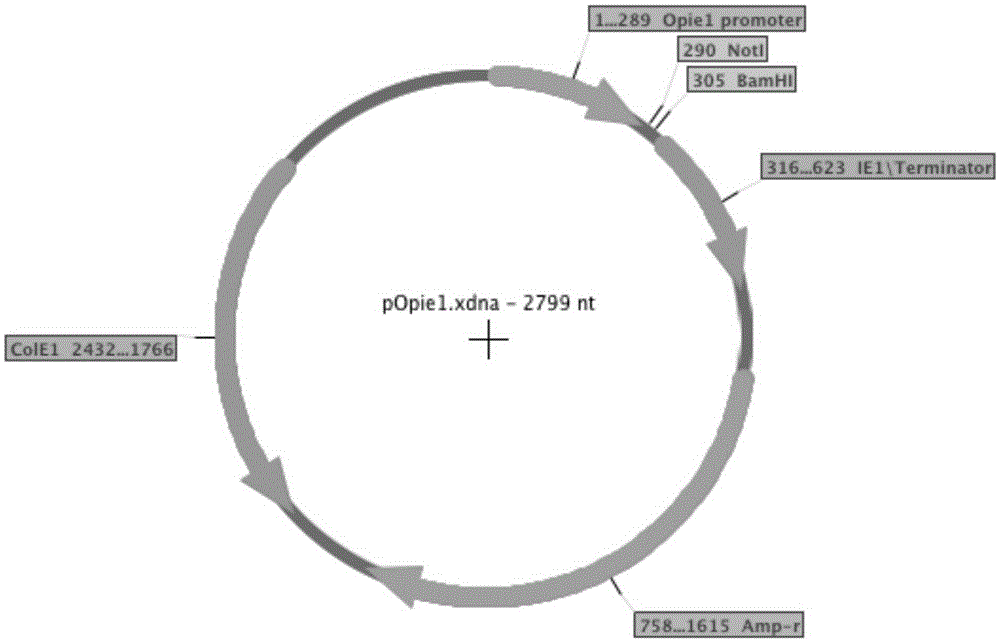
[0080]PIEx-XEGFP and PIEx-TNFR-Fc (Table 1) carry the genes of green fluorescent protein and human TNFR-Fc respectively, and are regulated by the Autographacalifornica multicapsidnucleopolyhedrovirus (AcMNPV) homology region 5 (hr5) enhancer and Lizao 1 (ie1) promoter controls, which have been described in a previous article (Shen et al., 2014). Using PIB / V5-His (Life Technologies) as a template, the promoters of Orgyiapseudotsugatamulticapsidnuclearpolyhedrosisvirus (OpMNPV) Opie1 (Opie1) and 2 (Opie2) were amplified by PCR using specific oligonucleotide primers (Table 2). Individual PCR products were digested with XmaI and NotI and subcloned into PIEx–XEGFP digested with the same two restriction enzymes, yielding pOPIE1-EGFP and pOPIE2-EGFP, respectively (Table 2). For these two plasmids, the AcMNPVhr5 enhancer and the iel promoter of PIEx-XEGFP could be replaced by Opie1 or Opie2 promoters, respectively. The metallothionein (MT) pro...
Embodiment 3
[0081] Example 3. Vector Construction
[0082] In the initial transfection of Tn-5 cells, we used an expression vector carrying the AcMNPVie1 promoter and hr5 enhancer. In order to study other promoters, we constructed expression vectors with Opie1, Opie2, and MT promoters, respectively. In addition, vectors carrying Opie1 promoter and AcMNPVhr5 enhancer, and vectors carrying Opie2 promoter and AcMNPVhr5 enhancer were also constructed. The vector carries either the EGFP gene or the TNFR-Fc gene and is constructed from the same starting vector PIEx-X (Shen et al., 2014). We also tested two other vectors: pUC-actEGFP, in which the EGFP gene is controlled by the Drosophila actin 5C promoter, and pMYKEF1-EGFP-puro, which is a vector carrying the EGFP gene (regulated by the hCMV- / enhancer control) mammalian expression vector. The pMYKEF1-EGFP-puro vector was reported to be efficiently expressed in Drosophila cells (Qin et al., 2010).
[0083] At the beginning, the cells were a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com