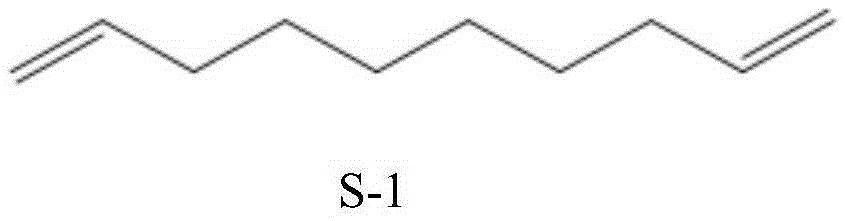
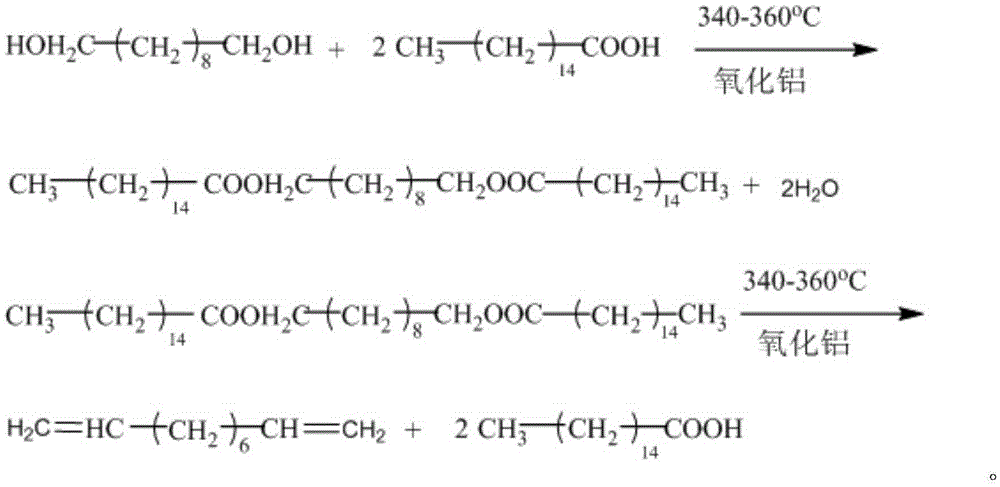Synthetic method of 1,9-decadiene
A synthesis method, the technology of decadiene, applied in the field of synthesis of 1,9-decadiene, can solve the problems of complex reaction conditions, high production cost, high reaction pressure, etc., and achieve good atom economy, fast reaction rate, cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, a kind of synthetic method of 1,9-decadiene, carries out following steps successively:
[0037] 1), in the 250mL four-neck flask that is equipped with a thermometer, a 10cm rectification column with a condensing reflux device installed on the top, a stirring paddle, and a heating dropping funnel, add 128g (0.5mol) palmitic acid, and γ-Al 2 o 3 (about 15g) as a catalyst, the molar ratio of the two is 1:0.3. After the temperature was raised to 340° C., 1,10-decanediol was continuously fed in a dropwise manner to react. The output speed is 40g / h, and the feed rate is adjusted so that it is basically consistent with the output speed. Continuous reaction for 6 hours, feed 250g, discharge about 240g. The remaining mixed solution in the four-necked flask can continue to be used as bottom material for continuous reaction.
[0038] 2), stop the reaction, carry out normal pressure rectification on 240g of the discharged material, collect the 99±0.5°C fraction, s...
Embodiment 10
[0050] Embodiment 10, the palmitic acid (hexadecanoic acid) in embodiment 1 is changed into higher fatty acid as described in table 2, and molar weight is constant. The rest are equal to Example 1.
[0051] The yields of the obtained products are shown in Table 2 below.
[0052] Table 2
[0053] higher fatty acid
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com