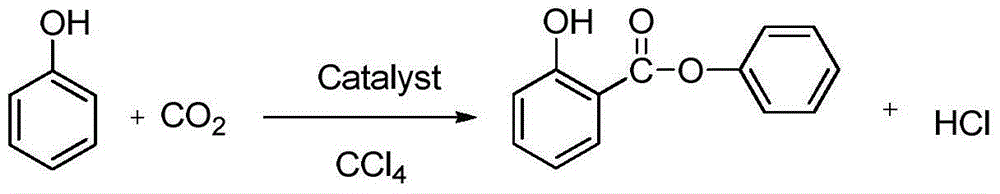
Method for one-step synthesis of phenyl salicylate from carbon dioxide and phenol
A technology of phenyl salicylate and carbon dioxide is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., and can solve the problems of a large amount of waste acid and waste gas, high corrosiveness of raw materials, and high production cost, and achieves Low cost, easy availability of raw materials, and the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 50mmol phenol and 50mLCCl to the 250mL reactor 4 , 20mol% ZnBr 2 , 30mmolEt 3 N, carbon dioxide with a pressure of 2.0 MPa was introduced into the reactor to replace the air in the reactor three times, the temperature was raised to 100 ℃, carbon dioxide was introduced into the reactor until the pressure in the reactor increased to 9.0 MPa, and the stirring was continued for 6.0 h, stop After stirring, the reaction mixture was cooled to room temperature, qualitatively analyzed by gas chromatography-mass spectrometry, and quantitatively analyzed by gas chromatography. The yield of phenyl salicylate was 4.4%.
Embodiment 2
[0024] Add 50mmol phenol and 50mLCCl to the 250mL reactor 4 , 50mol% ZnBr 2 , 30mmolEt 3 N. Feed carbon dioxide with a pressure of 2.0MPa into the reactor to replace the air in the reactor three times. The temperature is increased to 140°C, and carbon dioxide is fed into the reactor until the pressure in the reactor rises to 11.0MPa, and the stirring is continued for 8.0h to stop stirring. The reaction mixture was cooled to room temperature, qualitatively analyzed by gas chromatography-mass spectrometry, and quantitatively analyzed by gas chromatography. The yield of phenyl salicylate was 12.1%.
Embodiment 3
[0026] Add 50mmol phenol and 50mLCCl to the 250mL reactor 4 , 30mol% ZnCl 2 , 30mmolEt 3 N. Feed carbon dioxide with a pressure of 2.0MPa into the reactor to replace the air in the reactor three times. The temperature is raised to 125°C, and carbon dioxide is fed into the reactor until the pressure in the reactor rises to 9.0MPa. Continue to stir and react for 2.0h to stop stirring. The reaction mixture was cooled to room temperature, qualitatively analyzed by gas chromatography-mass spectrometer, and quantitatively analyzed by gas chromatography. The yield of phenyl salicylate was 2.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com