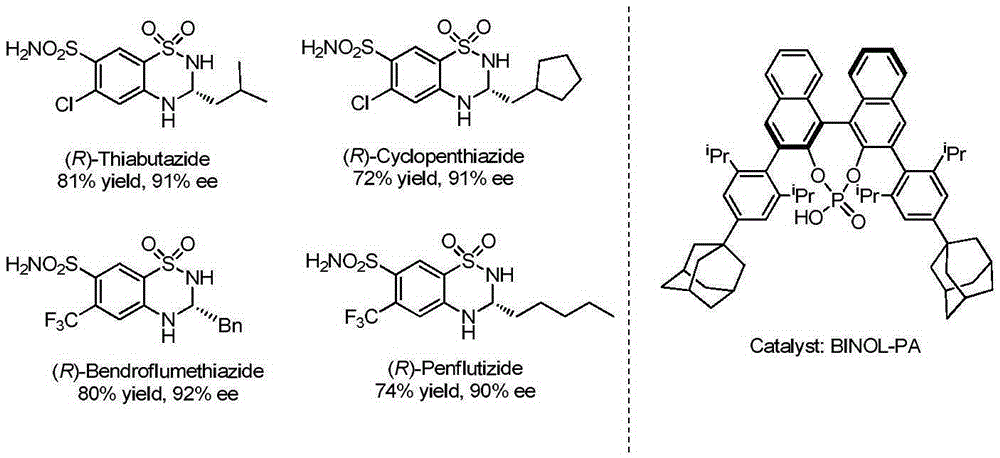
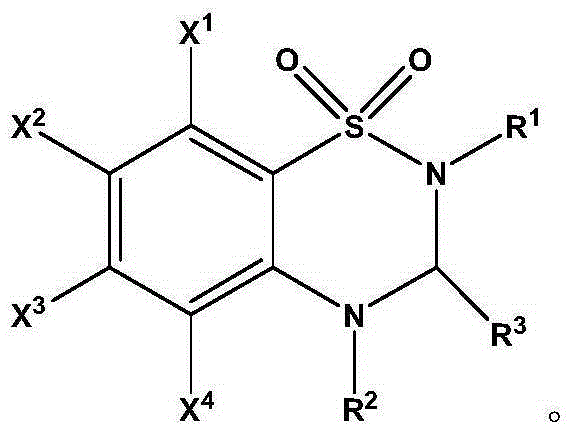
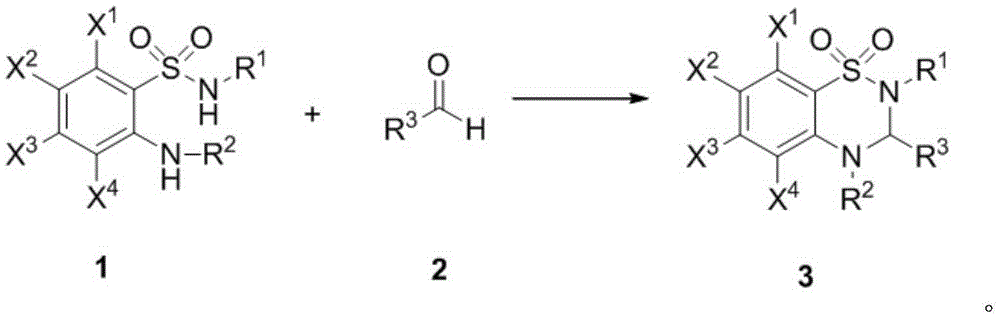
Thiadiazine compound and asymmetric synthetic method thereof
A synthesis method, thiadiazine technology, applied in the field of catalytic asymmetric synthesis of thiadiazine compounds, can solve the problems of long reaction time, high cost, cumbersome synthesis, etc., and achieve the effect of short reaction time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add Sc(OTf) to the Schlenk tube 3 (7.4mg, 0.015mmol), chiral ligand L1 (6.8mg, 0.0225mmol) and freshly distilled dichloromethane 1.0mL, react at room temperature for 1 hour, add anthranilamide (26mg, 0.15mmol), isopentyl Aldehyde (20mg, 0.225mmol) and 30mg Molecular sieves were reacted at -40°C for 24 hours and separated by silica gel column chromatography to obtain 13.7 mg of a white solid with a yield of 38% and an ee value of 66%. 1 HNMR (400MHz, DMSO) δ = 7.46 (dd, J 1 =8.0Hz,J 2 =1.2Hz,1H),7.40(d,J=11.2Hz,1H),7.31-7.27(m,1H),6.97(s,1H),6.82(d,J=8.0Hz,1H),6.71(dt ,J 1 =8.0Hz,J 2 =0.4Hz,1H),4.71(s,1H),1.92-1.85(m,1H),1.73-1.66(m,1H),1.62-1.55(m,1H),0.96-0.93(m,6H); 13 CNMR (100MHz, DMSO) δ=144.25, 133.16, 124.20, 121.77, 116.74, 116.45, 64.55, 42.54, 23.91, 23.18, 22.34.
Embodiment 2
[0024] Add Sc(OTf) to the Schlenk tube 3 (7.4mg, 0.015mmol), chiral ligand L3 (8.3mg, 0.0225mmol) and freshly distilled dichloromethane 1.0mL, react at room temperature for 1 hour, add anthranilamide (26mg, 0.15mmol), isopentyl Aldehyde (20mg, 0.225mmol) and 30mg Molecular sieves were reacted at -40°C for 24 hours and separated by silica gel column chromatography to obtain 18.0 mg of a white solid with a yield of 50% and an ee value of 83%.
Embodiment 3
[0026] Add Sc(OTf) to the Schlenk tube 3 (7.4mg, 0.015mmol), chiral ligand L5 (8.9mg, 0.0225mmol) and freshly distilled dichloromethane 1.0mL, react at room temperature for 1 hour, add anthranilamide (26mg, 0.15mmol), isopentyl Aldehyde (20mg, 0.225mmol) and 30mg Molecular sieves were reacted at -40°C for 24 hours and separated by silica gel column chromatography to obtain 21.2 mg of a white solid with a yield of 59% and an ee value of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com