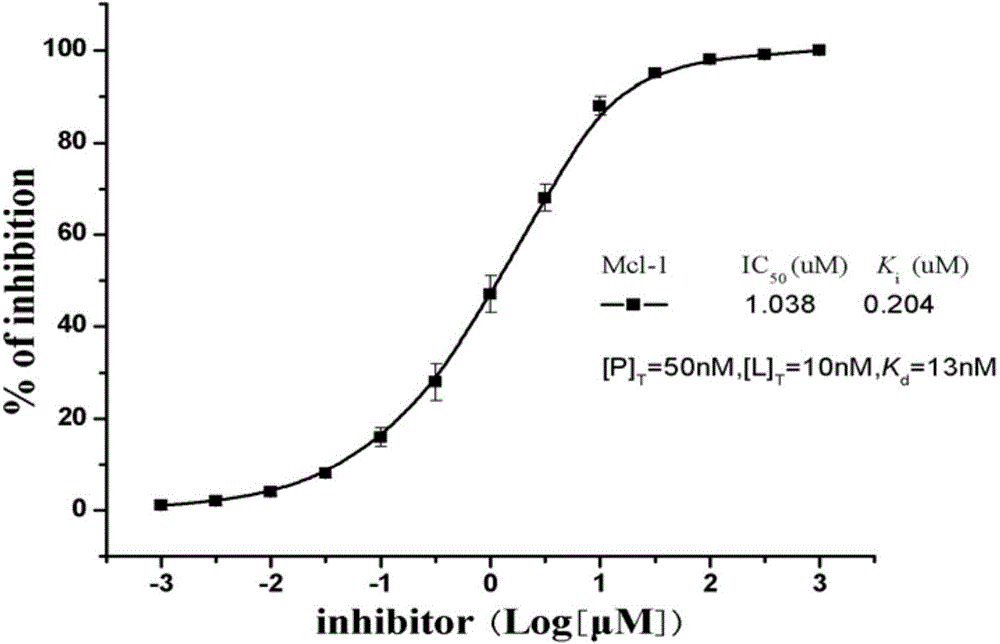
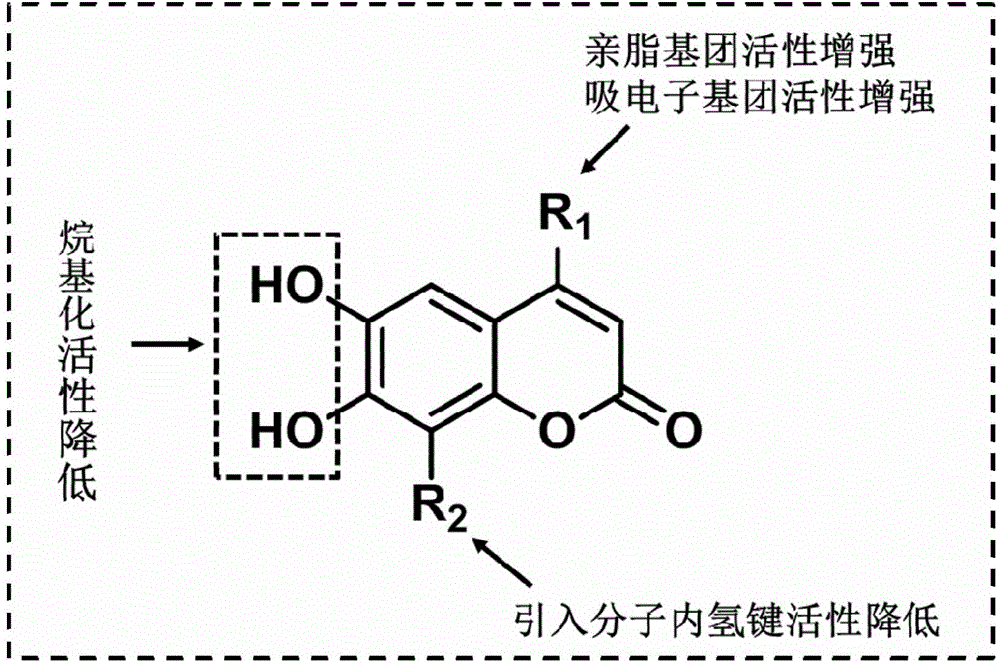
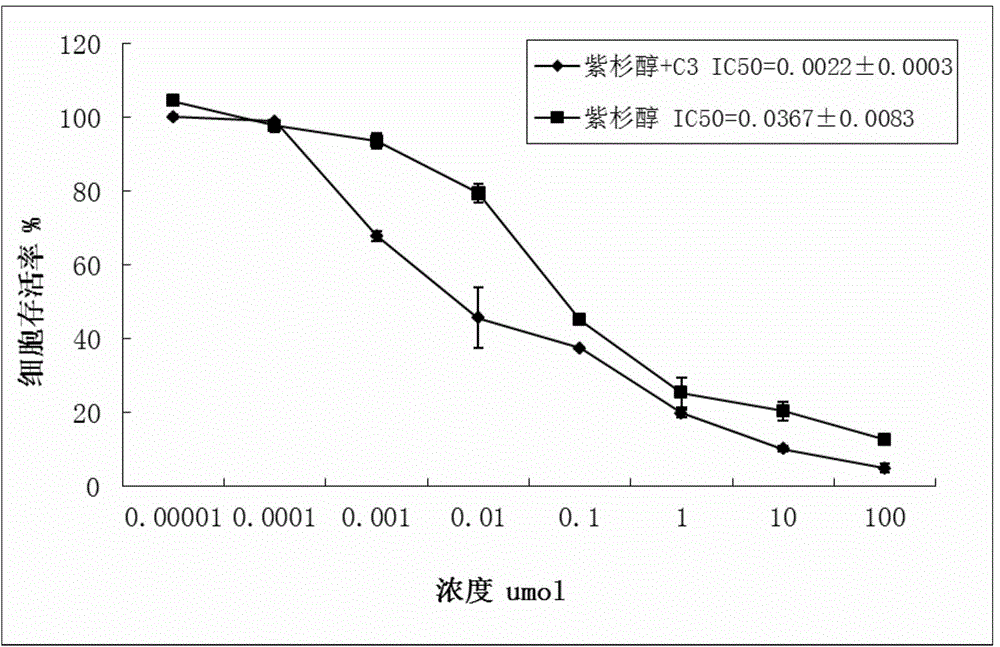
Anti-apoptosis Mcl-1 protein inhibitor, and applications thereof
A protein inhibitor, mcl-1 technology, applied in the field of medicine, can solve the problems of toxicity, short metabolic half-life, and low inhibitory activity, and achieve the effect of increasing inhibitory activity, good safety, and reversing multidrug resistance of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of 6,7-dihydroxy-4-methylcoumarin (C-2)
[0023]
[0024] Weigh 1.5g of 1,2,4-triacetoxybenzene into a three-neck flask, add 1ml of ethyl acetoacetate, 5ml of perchloric acid, stir at room temperature for 20 minutes, heat to 80°C, react for 3h, and detect the reaction by TLC At the end, the reaction solution was slowly poured into 30ml of ice water, stirred for 30min, filtered, and the filter cake was collected, which was the crude product. The crude product was dissolved in 20ml of methanol, heated to reflux, cooled to room temperature after 1h, filtered, and the filter cake was collected and vacuum-dried to obtain 1.1g of compound C-2 as a milky white solid.
[0025] The NMR test results are as follows:
[0026] 1 HNMR(400MHz,d-DMSO)δ:2.31(s,3H,CH 3 ),6.09(s,1H,C=CH),6.73(s,1H,ArH),7.00(s,1H,ArH),9.34(s,br,1H,ArOH),10.19(s,br,1H, ArOH);
[0027] 13 CNMR (100MHz, d-DMSO) δ: 18.68, 103.13, 109.89, 110.86, 111.96, 143.24, 148.17, 150.58, 153.68, 161....
Embodiment 2
[0029] Preparation of 6,7-dihydroxy-4-trifluoromethylcoumarin (C-3)
[0030]
[0031] Weigh 1.5g of 1,2,4-triacetoxybenzene into a three-necked flask, add 1.4ml of ethyl trifluoroacetoacetate, 5ml of perchloric acid, stir at room temperature for 20 minutes, heat to 80°C, and react for 3h. The reaction was detected by TLC, and the reaction solution was slowly poured into 30 ml of ice water, stirred for 30 min, filtered, and the filter cake was collected, which was the crude product. The crude product was dissolved in 20ml of methanol, heated to reflux, cooled to room temperature after 1h, filtered, the filter cake was collected, and dried in vacuo to obtain 1.3g of compound C-3 as a light yellow solid. The NMR test results are as follows:
[0032] 1 HNMR (400MHz, d-DMSO) δ: 6.71(s, 1H, C=CH), 6.86(s, 1H, ArH), 7.03(s, 1H, ArH), 9.78(s, br, 1H, ArOH), 10.61(s,br,1H,ArOH);
[0033] 13 CNMR (100MHz, d-DMSO) δ: 104.01, 105.05, 108.89, 112.12, 120.94, 123.68, 144.03, 149.59,...
Embodiment 3
[0035] Preparation of 6,7-dihydroxy-4-chloromethylcoumarin (C-4)
[0036]
[0037]Weigh 1.5g of 1,2,4-triacetoxybenzene into a three-neck flask, add 1.3ml of ethyl 4-chloroacetoacetate, 5ml of perchloric acid, stir at room temperature for 20 minutes, heat to 80°C, and react for 3h , TLC detection of the end of the reaction, the reaction solution was slowly poured into 30ml of ice water, stirred for 30min, filtered, and the filter cake was collected, which was the crude product. The crude product was dissolved in 20ml of methanol, heated to reflux, cooled to room temperature after 1h, filtered, and the filter cake was collected and dried in vacuo to obtain 1.2g of compound C-3 as a milky white solid.
[0038] The NMR test results are as follows:
[0039] 1 HNMR(400MHz,d-DMSO)δ:4.90(s,2H,CH 2 Cl),6.40(s,1H,C=CH),6.78(s,1H,ArH),7.12(s,1H,ArH),9.48(s,br,1H,ArOH),10.39(s,br,1H ,ArOH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com