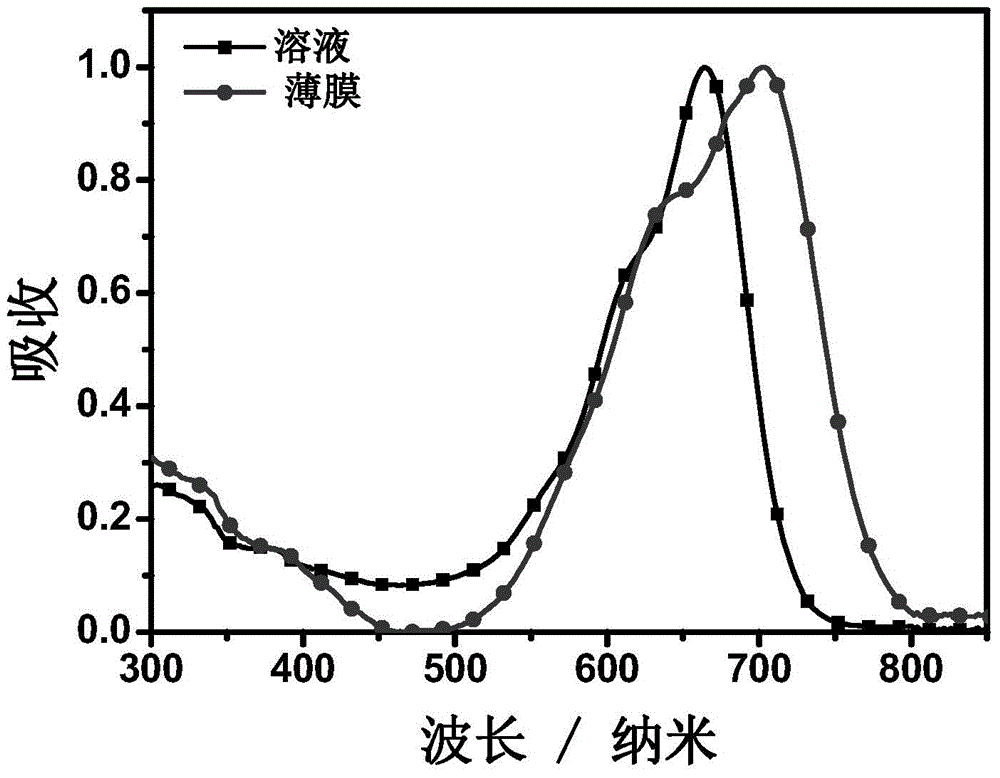
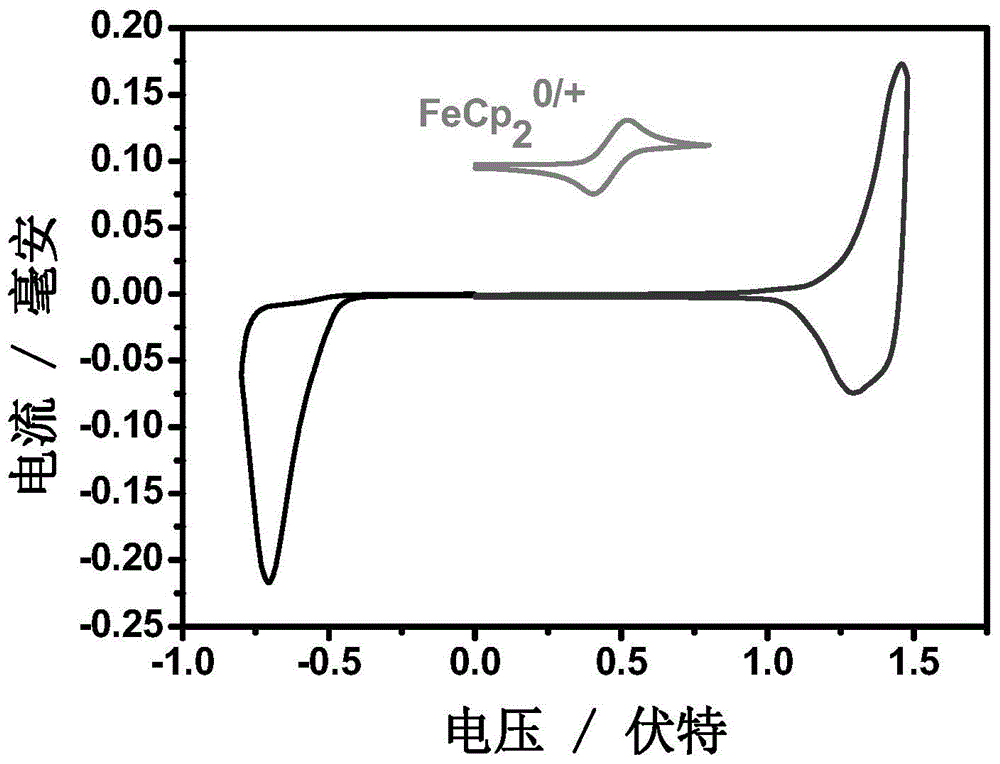
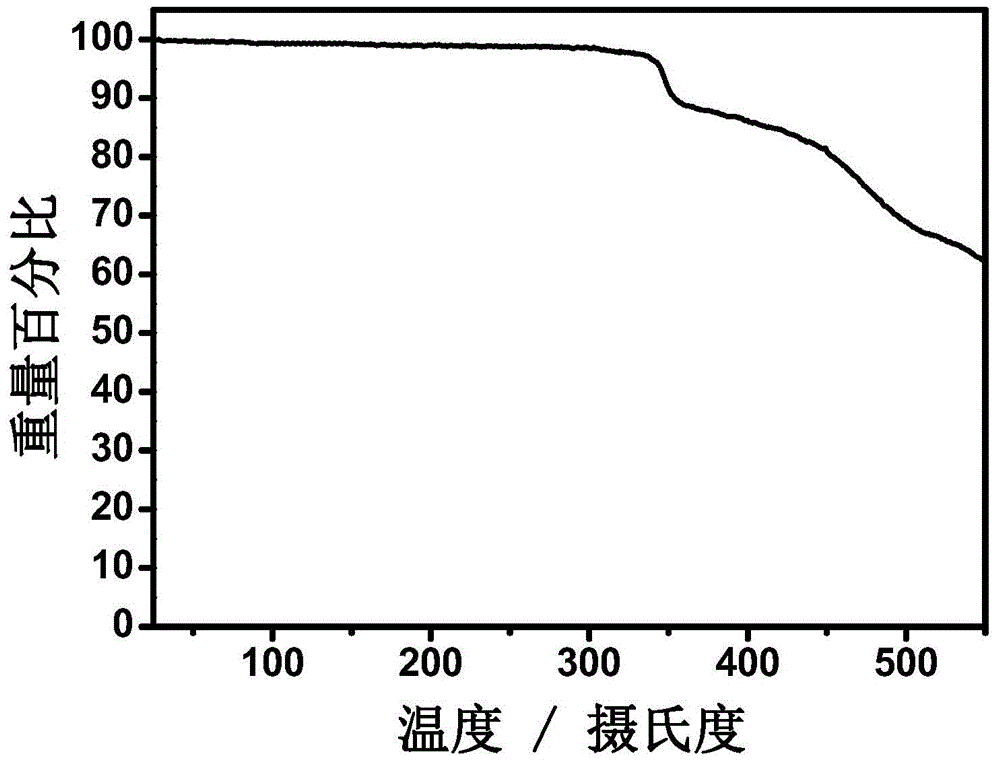
A-D-A conjugated molecules based on hepta-condensed ring units and preparation method for A-D-A conjugated molecules and application of A-D-A conjugated molecules
A technology of A-D-A and conjugated molecules, which is applied in the fields of electrical components, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., to achieve the effects of strong visible light absorption, good thermal stability, and high charge transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The synthetic route of A-D-A conjugated molecule 1 based on hepta-fused ring unit is as follows:
[0061]
[0062] Add compound a (200mg, 0.19mmol) in the three-necked round-bottomed flask, compound b (279mg, 1.4mmol), chloroform (50mL), add 1mL pyridine after logical argon 30 minutes to remove the air in the three-necked round-bottomed flask; Stir the reaction at 65°C for 12 hours, then cool to room temperature; pour the resulting reaction solution into 200mL of methanol, filter the precipitate obtained and dry it with silica gel (200-300 mesh) column chromatography for separation, the eluent is petroleum Ether / dichloromethane (volume ratio 1:1), the product was a dark blue solid (57 mg, 21%), which was the A-D-A conjugated molecule 1 based on hepta-fused ring units. 1 HNMR (400MHz, CDCl 3 ):δ8.87(s,2H),8.70(d,J=7.6Hz,2H),8.22(s,2H),7.93(d,J=6.4Hz,2H),7.79(m,4H),7.63 (s,2H),7.23(d,J=8.4Hz,8H),7.15(d,J=8.4Hz,8H),2.58(m,8H),1.61(m,8H),1.33(m,24H) ,0.87(m,12H). 13 ...
Embodiment 2
[0065] The synthetic route of A-D-A conjugated molecule 2 based on hepta-fused ring unit is as follows:
[0066]
[0067] Add compound c (165mg, 0.11mmol), compound b (219mg, 1.1mmol), chloroform (50mL) in the three-necked round-bottomed flask, and add 0.5mL of pyridine after argon flow for 30 minutes to remove the air in the three-necked round-bottomed flask; Stir the reaction at a temperature of 65°C for 12 hours, then cool to room temperature; pour the resulting reaction solution into 200mL of methanol, filter the precipitate obtained and dry it with silica gel (200-300 mesh) column chromatography, the eluent is Petroleum ether / dichloromethane (volume ratio 1:1), the product was a dark green solid (152 mg, 74%), which was the A-D-A conjugated molecule 2 based on hepta-fused ring units. 1 HNMR (400MHz, CDCl 3 ):δ8.78(s,2H),8.70(d,J=7.2Hz,2H),7.93(d,J=7.2Hz,2H),7.77(m,4H),7.69(s,2H),7.60 (s,2H),7.56(s,2H),7.21(d,J=8.0Hz,8H),7.14(d,J=8.0Hz,8H),2.78(d,J=7.2Hz,4H),2.60 (m,...
Embodiment 3
[0070] The synthetic route of A-D-A conjugated molecule 3 based on hepta-fused ring unit is as follows:
[0071]
[0072] Add compound d (200mg, 0.148mmol), compound e (100mg, 0.345mmol) and 20mL toluene in the three-necked round-bottomed flask, pass argon gas 15 minutes to remove the air in the three-necked round-bottomed flask, add Pd (PPh 3 ) 4 (40mg, 0.034mmol), stirred at a temperature of 110 ° C for 48 hours; cooled to room temperature; added 40mL (0.1gmL -1 ) KF aqueous solution, stirring overnight at room temperature, to remove tin impurities; add 150mL water in the resulting reactant, CH 2 Cl 2 (2 × 150mL) for extraction, the resulting organic phase with anhydrous MgSO 4 Carry out drying; After filtering and spinning to remove the solvent, use petroleum ether / dichloromethane mixed solvent (volume ratio 1:1) as eluent, silica gel (200~300 mesh) column chromatography separation and purification to obtain a blue-black solid ( 30 mg, 14.1%), which is the A-D-A conj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com