Silver electrolysis device and process
A process and electrolytic cell technology, applied in the field of silver electrolysis devices and processes, can solve the problems of electrolyte pollution, low electrolysis efficiency, complex electrolytic cell structure, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
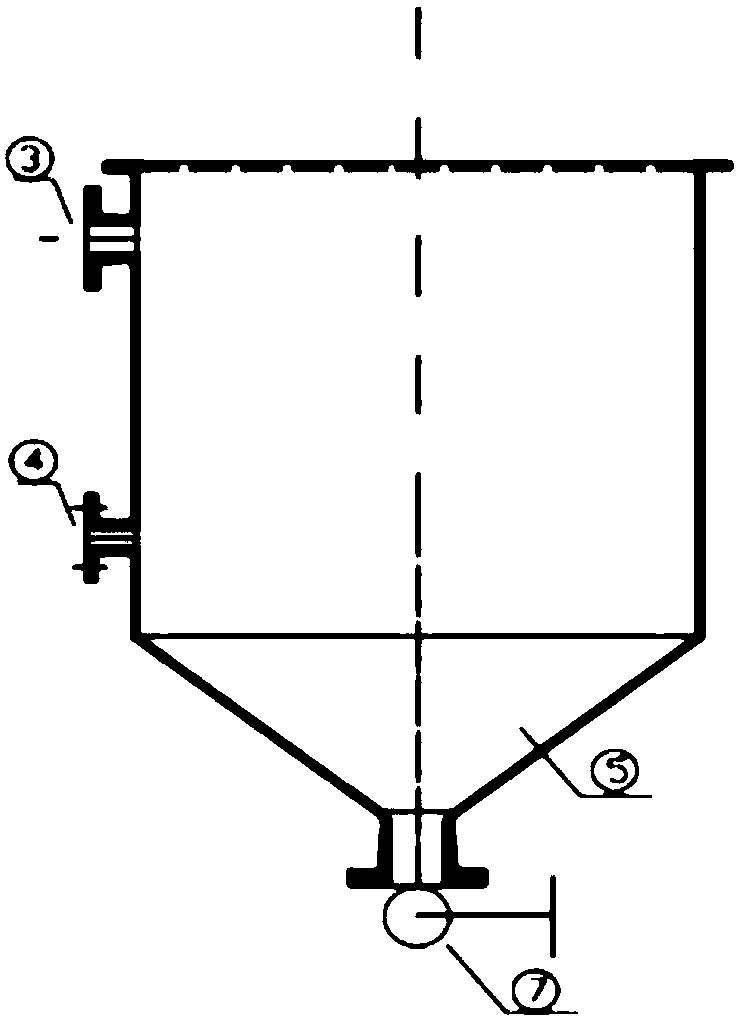
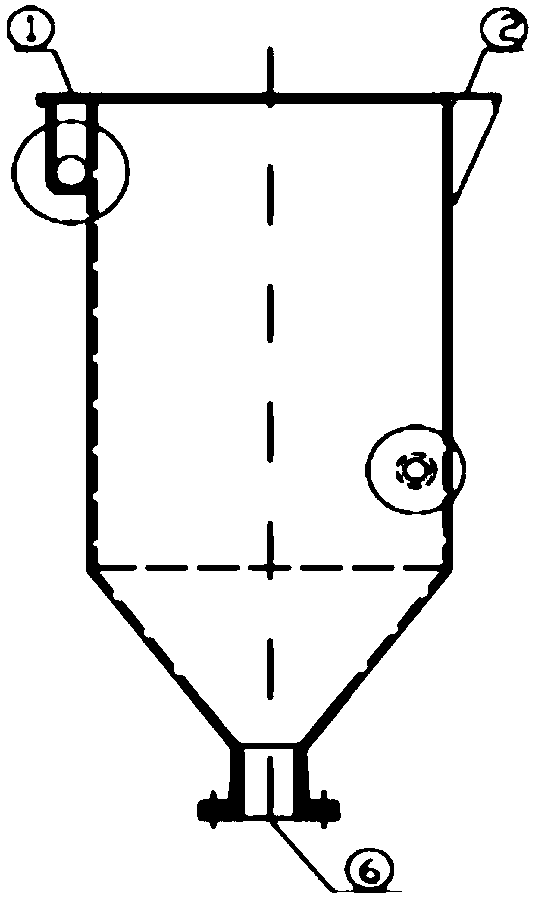
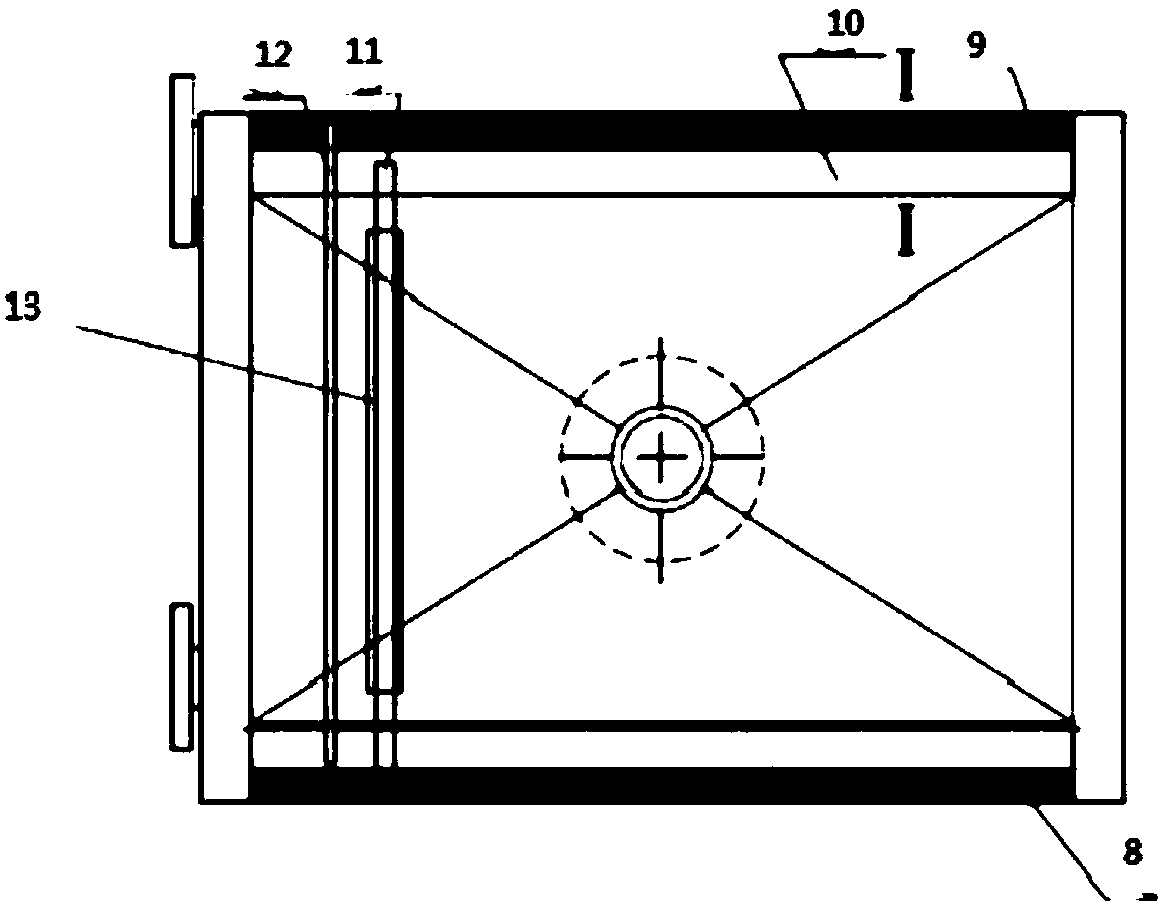
[0074] The electrolytic cell uses 98% gold-silver alloy as the anode, wherein the gold content is 10%, and stainless steel is the negative electrode. The length of the cuboid upper part of the electrolytic cell is 800 mm, the width is 500 mm, and the height is 750 mm. The bottom of the electrolytic cell is 270 mm high. The electrolytic cell is provided with 6 anode plates with a length of 440 mm and a width of 390 mm and 7 cathode plates with a length of 460 mm and a width of 410 mm, and the distance between the anode plate and the cathode plate is 100 mm.
[0075] Electrolysis parameters: the concentration of silver nitrate is 120g / L, the flow rate of the electrolyte inlet is 2m / s, the voltage is 3.5V, and the current intensity is 1200A / m 2 , the electrolysis pH value is 2.5, the electrolysis temperature is 45°C, and the electrolysis time is 16h. After electrolysis, silver is obtained by washing and smelting.
[0076] After calculation, the silver recovery rate is 99.99%, the...
Embodiment 2
[0078] The electrolytic cell uses 97% gold-silver alloy as the anode, wherein the gold content is 10%, and stainless steel is the negative electrode. The length of the cuboid upper part of the electrolytic cell is 1000 mm, the width is 625 mm, and the height is 940 mm. The bottom of the electrolytic cell is 340 mm high. The electrolytic cell is provided with 8 anode plates with a length of 545 mm and a width of 500 mm and 9 cathode plates with a length of 565 mm and a width of 520 mm, and the distance between the anode plate and the cathode plate is 100 mm.
[0079] Electrolysis parameters: the concentration of silver nitrate is 130g / L, the flow rate of the electrolyte inlet is 2.1m / s, the voltage is 4V, and the current intensity is 1300A / m 2 , the electrolysis pH value is 3, the electrolysis temperature is 50°C, and the electrolysis time is 17h. After electrolysis, silver is obtained by washing and smelting.
[0080] After calculation, the silver recovery rate is 99.99%, the ...
Embodiment 3
[0082] The electrolytic cell uses 95% gold-silver alloy as the anode, wherein the gold content is 10%, and stainless steel is the negative electrode. The length of the cuboid upper part of the electrolytic cell is 1200mm, the width is 750mm, and the height is 1120mm. The bottom of the electrolytic cell is 400mm high. The electrolytic cell is provided with 10 anode plates with a length of 690 mm and a width of 600 mm and 11 cathode plates with a length of 710 mm and a width of 620 mm, and the distance between the anode plate and the cathode plate is 100 mm.
[0083] Electrolysis parameters: the concentration of silver nitrate is 150g / L, the flow velocity of the electrolyte inlet is 2.2m / s, the voltage is 4.5V, and the current intensity is 1400A / m 2 , the electrolysis pH value is 3.5, the electrolysis temperature is 55°C, and the electrolysis time is 16 hours. After electrolysis, silver is obtained by washing and smelting.
[0084] After calculation, the silver recovery rate is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com