Tacrine-8-hydroxyl(amine)quinoline derivative and application thereof
A technology of tacrine and quinoline, applied in the field of biomedicine, can solve the problems of irreversible or curable diseases, high liver toxicity, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
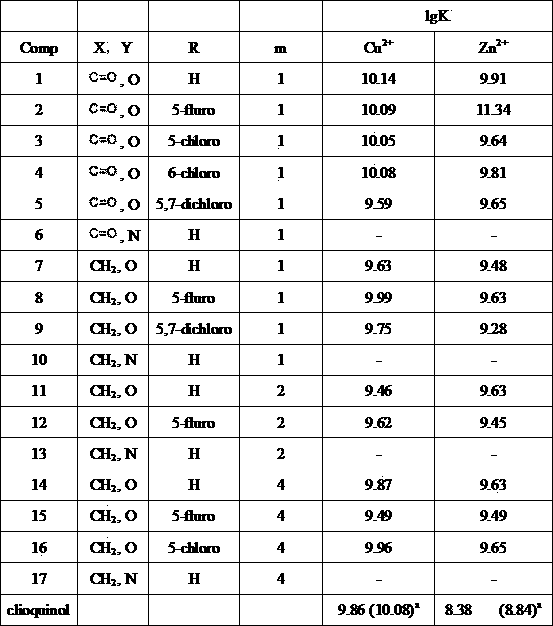
[0057] Synthesis of 2-[(4-(8-hydroxyquinoline)-2-methyl)piperidine]-N-(1,2,3,4-tetrahydroacridin-9-amino)acetamide 1
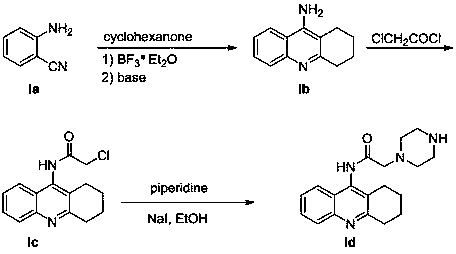
[0058] Weigh compound ID (0.5mmol) and compound In (0.5mmol, R=H) in a 25mL round bottom bottle, add NaBH(OAc) 3 (1.0mmol), DCE (dry, 5mL), heated to 50°C under the protection of argon to react, TLC monitored the reaction, cooled the reaction solution to room temperature, washed with water, extracted with DCM, concentrated to dryness and separated by silica gel column, the obtained product was added 37%HCl (2mL), heat to reflux for 1h, add water after cooling, K 2 CO 3 Adjusted to weakly alkaline, concentrated to dryness after extraction with DCM and separated by silica gel column to obtain the compound 1 , white solid, yield 66%.
[0059] 1 (400MHz, CDCl 3 )δ=9.22(s,1H),8.14(d, J =10.0Hz,1H),8.00(d, J =8.0Hz,1H),7.71(d, J =8.4Hz,1H),7.66–7.61(m,2H),7.48–7.41(m,2H),7.32(d, J =8.4Hz,1H),7.16(d, J =7.6Hz,1H),3.88(s,2H),3.33(s,2H),3.15(t, J =6.4...
Embodiment 2
[0061] Synthesis of 2-[(4-(5-chloro-8-hydroxyquinoline)-2-methyl)piperidine]-N-(1,2,3,4-tetrahydroacridin-9-amino)ethyl Amide 2
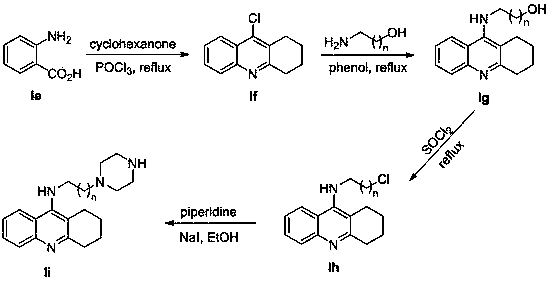
[0062] compound ID (0.5mmol) and compound In (0.5mmol, R=5-Cl) With reference to the operation process of Example 1, the compound was isolated 2 , white solid, yield 63%.
[0063] 1 (400MHz, CDCl 3 )δ=9.21(s,1H),8.49(d, J =8.8Hz,1H),8.00(d, J =8.4Hz,1H),7.76–7.70(m,2H),7.63(t, J =7.2Hz,1H),7.49–7.45(m,2H),7.09(d, J =8.8Hz,1H),3.89(s,2H),3.31(s,2H),3.15(t, J =6.4Hz,2H),2.85–2.80(m,6H),2.70(brs,4H),2.01–1.86ppm(m,4H); 13 CNMR (100MHz, CDCl 3 )δ=168.6,159.9,157.6151.2,146.9,138.1,137.9,133.9,128.9,128.8,127.1,126.0,125.5,123.7,122.5,121.8,120.4,110.2,64.3,61.30.8,552.3,75 ,22.5ppm;purity:99.6%,determined byHPLCwithInertSustainC18column(5μm,4.6×150mm)at254nm[CH 3 CN(contain0.05%CF 3 CO 2 H) / H 2 O=95:5, flowrate=1.2mL / min], t R =1.37min;ESI-MSm / z:538.3[M+Na] + .
Embodiment 3
[0065] Synthesis of 2-[(4-(5-fluoro8-hydroxyquinoline)-2-methyl)piperidine]-N-(1,2,3,4-tetrahydroacridin-9-amino)acetamide 3
[0066] compound ID (0.5mmol) and compound In (0.5mmol, R=5-F) with reference to the operation process of Example 1, the compound was isolated 3 , white solid, yield 60%.
[0067] 1 (400MHz, CDCl 3 )δ=9.21(s,1H),8.38(d, J =8.8Hz,1H),8.00(d, J =8.4Hz,1H),7.74–7.70(m,2H),7.65–7.61(m,2H),7.63(t, J =7.6Hz,1H),7.47(t, J =7.6Hz,1H),7.13–7.03(m,2H),3.88(s,2H),3.33(s,2H),3.15(t, J =6.4Hz,2H),2.86–2.80(m,6H),2.71(brs,4H),2.01–1.97(m,2H),1.90–1.86ppm(m,2H); 13 CNMR (100MHz, CDCl3 )δ=168.6,159.9,158.0,150.7( J C-F =244.1Hz), 148.2( J C-F =2.7Hz),147.0,138.0,137.1,137.0,130.3( J C-F =3.1Hz), 129.0, 128.9, 127.1, 125.9, 123.7, 121.8, 121.8 ( J C-F =1.9Hz), 118.0( J C-F =18.7Hz), 110.4( J C-F =20.5Hz), 108.8( J C-F =7.1Hz),64.5,61.8,54.0,53.5,34.0,25.8,22.7,22.5ppm;purity:94.2%,determined byHPLCwithInertSustainC18column(5μm,4.6×150mm)at2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com