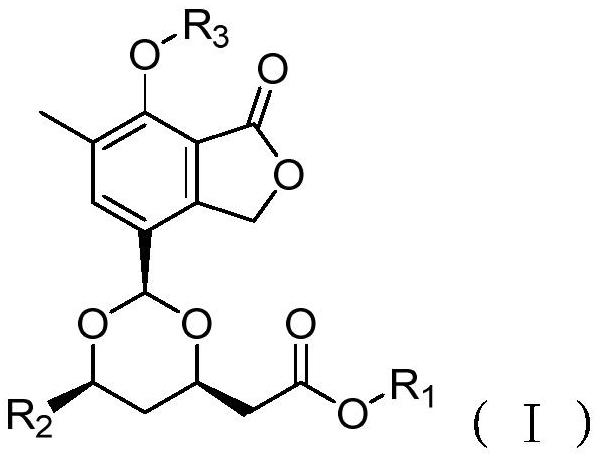
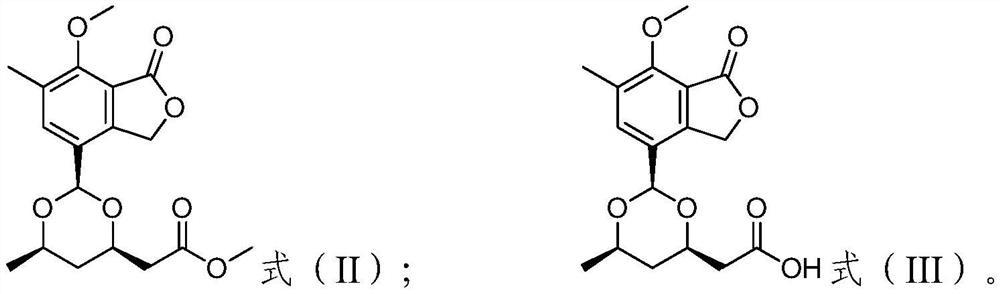
A kind of carbon skin phthalide compound, composition, preparation method and use thereof
A technology of compounds and phthalides, applied in the field of natural product drugs, can solve the problem of less drug reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Mass fermentation of charcoal fungus 69-8-7-1 and its sample pretreatment method:
[0056] (1) The fungus 69-8-7-1 of the genus Charcoal was inoculated into the PDB medium after being activated by the PDA slant, and was incubated at 25°C for 200 r.min. -1 The seed liquid was prepared by shaking culture for 5 days, and then the seed liquid was inoculated into 20 flasks containing rice culture medium according to the inoculation amount of 5 mL / bottle, and the culture was statically cultured at 25° C. for 48 days to obtain a fermented product. Described rice culture medium is made up of following components: rice 70g / bottle, purified water 105L / bottle.
[0057] (2) The fermented product was added to ethyl acetate for immersion extraction 3 times, and the extract was concentrated to dryness under reduced pressure to obtain a crude extract (30.5 g).
Embodiment 2
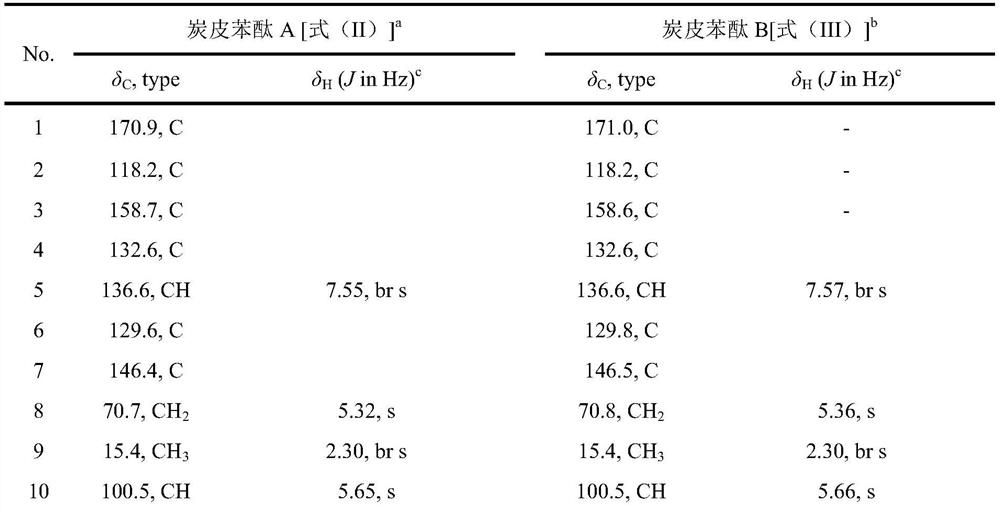
[0059] Preparation of carbon skin phthalide A and carbon skin phthalide B:
[0060] The crude extract was passed through a silica gel column and eluted with cyclohexane and methanol to obtain a cyclohexane fraction c (9.6 g) and a methanol fraction w (16.5 g); then the methanol fraction w was subjected to medium and low pressure ODS column chromatography, followed by 4 fractions (w1, w2, w3, w4) were obtained by gradient elution of methanol-water with volume ratios of 20:80, 50:50, 70:30 and 100:0; and methanol-water washing with a volume ratio of 70:30 The obtained sub-fraction w2 (2.4g) was subjected to medium and low pressure liquid phase ODS column chromatography, followed by volume ratios of 10:90, 15:85, 20:80, 25:75, 30:70, 35:65, 40: 60, 45:55, 50:50, 100:0 methanol-water gradient to give w2-1,w2-2,w2-3,w2-4,w2-5,w2-6,w2-7,w2- 8 and w2-9 make a total of 9 subfractions. Subfraction w2-9 (871.4 mg) was prepared by reverse-phase HPLC (Phenomenex Gemini C8 column), elute...
Embodiment 3
[0073] Test method for acetylcholinesterase activity of compounds
[0074] (1) Experimental principle
[0075] AChE catalyzes the hydrolysis of the substrate thioacetylcholine iodide to acetic acid and thiocholine iodide. Thiocholine iodide reacts with 5,5-thio-bis-2-nitrobenzoic acid (5,5-dithio-bis-2-nitrobenzoic acid, DTNB) to generate the yellow anion 5-thio-2- Nitrobenzoic acid, which has the maximum absorption at 405nm, can reflect the activity of AChE by measuring its maximum generation amount in unit time.
[0076] (2) Preparation of solutions and reagents for experiments
[0077] Weigh 2.622g NaH 2 PO 4 ·H 2 O and 21.714g Na 2 HPO 4 ·7H 2 O, mix them and add water to 1000mL, adjust the pH to 7.40 with HCl, and then prepare 1 mol / L phosphate buffer (PB). Weigh 27.4 mg of DTNB, dissolve it in 100 mL of PB, and prepare DTNB with a concentration of 0.7 mM, protect from light, and store at below 4°C. 8.7 mg of thioacetylcholine iodide (ATCh) was dissolved in 10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com