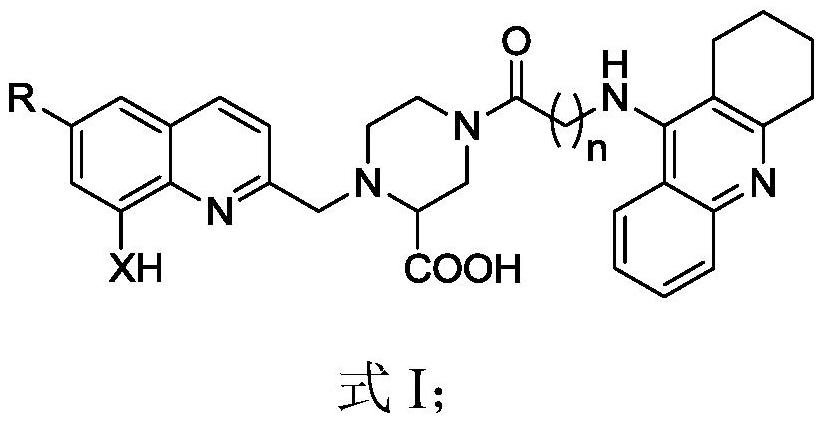
A 2-carboxypiperazine-linked tacrine-8-amino(hydroxy)quinoline derivative and its preparation and application
A technology of tacrine and its derivatives, which is applied in the field of tacrine-8-aminoquinoline derivatives and their preparation and application, can solve the problems of urgent, high liver toxicity, low bioavailability, etc. Delayed Aggregation and Effective Treatment of Alzheimer's Disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
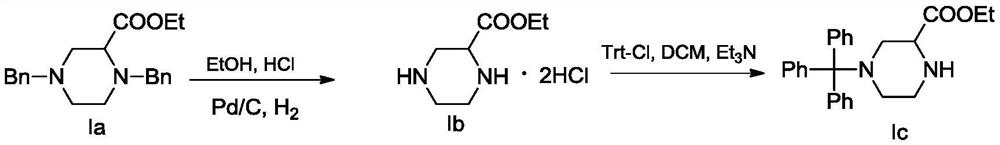
[0061] (1) Synthetic compound Ia 1,4-dibenzylpiperazine-2-carboxylic acid ethyl ester
[0062] Weigh N,N'-dibenzylethylenediamine (50g, 208.04mmol) into a 1000mL round-bottomed flask containing 300mL of toluene, then add triethylamine (72mL, 520.1mmol) and keep stirring, 2, Ethyl 3-dibromopropionate (54.08g, 208.04mmol) was dissolved in 50mL of toluene, and then slowly poured into a flask. After the reaction system was stirred and reacted at 25°C for 30min, the toluene was removed by concentration under reduced pressure, and water and di Extract and separate the organic phase with methyl chloride, wash the organic phase with water once, then dry with anhydrous magnesium sulfate, filter, and concentrate under reduced pressure; the crude product is separated and purified by silica gel column chromatography to obtain compound Ia 1,4-dibenzylpiperene in yellow oil Ethylazine-2-carboxylate, 85% yield.
[0063] 1 H NMR (400MHz, CDCl3): δ=7.31–7.29(m,2H),7.26–7.20(m,6H),7.19–7.15(m...
Embodiment 2
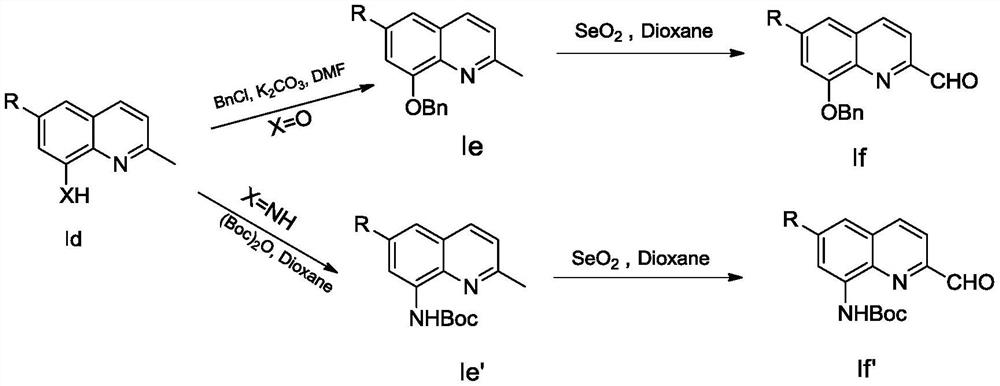
[0071] (1) Synthetic compound Id 6-fluoro-2-methylquinolin-8-alcohol (X=O, R=F)
[0072] 2-amino-5-fluorophenol (20g, 157.3mmol) and acetaldehyde (27.7g, 629.3mmol) in concentrated hydrochloric acid (140mL), 80 ℃ condensation cyclization reaction 2.5h; The product Id6-fluoro-2-methylquinolin-8-ol (X=O, R=F) was isolated as a black oil in 9% yield.
[0073] 1 H NMR (400MHz, CDCl3): δ = 7.95 (d, J = 8.4Hz, 1H), 7.31 (d, J = 8.4Hz, 1H), 6.94-6.88 (m, 2H), 2.69 (s, 3H); ESI-MSm / z:178.1[M+H] +
[0074] (2) Synthetic compound Id 6-fluoro-2-methylquinolin-8-amine (X=NH, R=F)
[0075] 4-Fluoro-2-nitroaniline (2g, 12.8mmol) and acetaldehyde (1.8g, 25.6mmol) were cyclized by condensation in hydrochloric acid (6M, 50mL) and toluene (20mL) at 80°C for 3h, separated by silica gel column The purified product (3g, 14.5mmol) was reacted with reduced iron powder (2.4g, 43.6mmol) in a mixed solution of acetic acid (30mL) and ethanol (50mL) for 2h under reflux; after the reaction was monito...
Embodiment 3
[0078] (1) Synthetic compound Ie 8-benzyloxy-2-methylquinoline (R=H)
[0079] Weigh compound Id 2-methylquinolin-8-ol (X=O, R=H, direct purchase) (5g, 31.4mmol) and potassium carbonate (6.5g, 47.1mmol) in a round bottom flask (50mL) Add benzyl bromide (3.8mL, 31.4mmol) and N,N-dimethylformamide (20mL) sequentially, heat the reaction system to 80°C and stir for 10h; after the reaction system is cooled, add water and ethyl acetate Extracted 3 times, the organic phase was washed again with water, dried with anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure; the crude product was separated and purified by silica gel column chromatography to obtain a white solid Ie8-benzyloxy-2-methylquinoline (R = H), the yield is 98%.
[0080] 1 H NMR (400MHz, CDCl3): δ = 8.00 (d, J = 8.4Hz, 1H), 7.52 (d, J = 7.2Hz, 2H), 7.40–7.27 (m, 6H), 7.01 (dd, J = 7.6 Hz,1.6Hz,1H),5.46(s,2H),2.81(s,3H); ESI-MSm / z:251.1[M+H] + .
[0081] (2) Synthetic compound Ie 8-benzyloxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com