Anti-HER3 antibody, preparing method thereof and applications of the antibody
A technology of antibody and antibody heavy chain, which is applied in the field of biomedicine and can solve problems such as lack of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1, Preparation of HER3 mouse monoclonal antibody
[0153] 1. Preparation of HER3 monoclonal antibody hybridoma cell lines
[0154] 1. Immunogen
[0155] The immunogen is the full-length phenylalanine extracellular region (HER3-ECD-H584F) whose histidine at position 584 in the extracellular region of HER3 is mutated by CHO / dhfr - Cells (purchased from ATCC) were stably expressed, and the supernatant of cell culture was purified.
[0156] 2. Immunization of Balb / c mice
[0157] Balb / c mice were purchased from Shanghai Slack Co., Ltd. All Balb / c mice used for immunization were 3-week-old, female, standardized disease-free, healthy purebred mice, which met the US FDA standards.
[0158] 3. Balb / c mouse immunization method
[0159] The first immunization: Mix 50 μg (250 μl) antigen with 250 μl MF59 adjuvant, prepare a 500 μl solution, and inject it into Balb / c mice at multiple points and paws subcutaneously. The second immunization: three weeks after the first...
Embodiment 2
[0178] Embodiment 2, mouse monoclonal antibody affinity determination
[0179] The affinity of the HER3 monoclonal antibody was determined using the proteonTMXPR36ProteinInteractionArraySystem method (refer to BronnerV, DenkbergG, PeledM, et al. Therapeutic antibodies: Discovery and development using the ProteOnXPR36biosensorinteractionarraysystem. AnalBiochem2010; 406: 147-156).
[0180] Antibody number
Embodiment 3
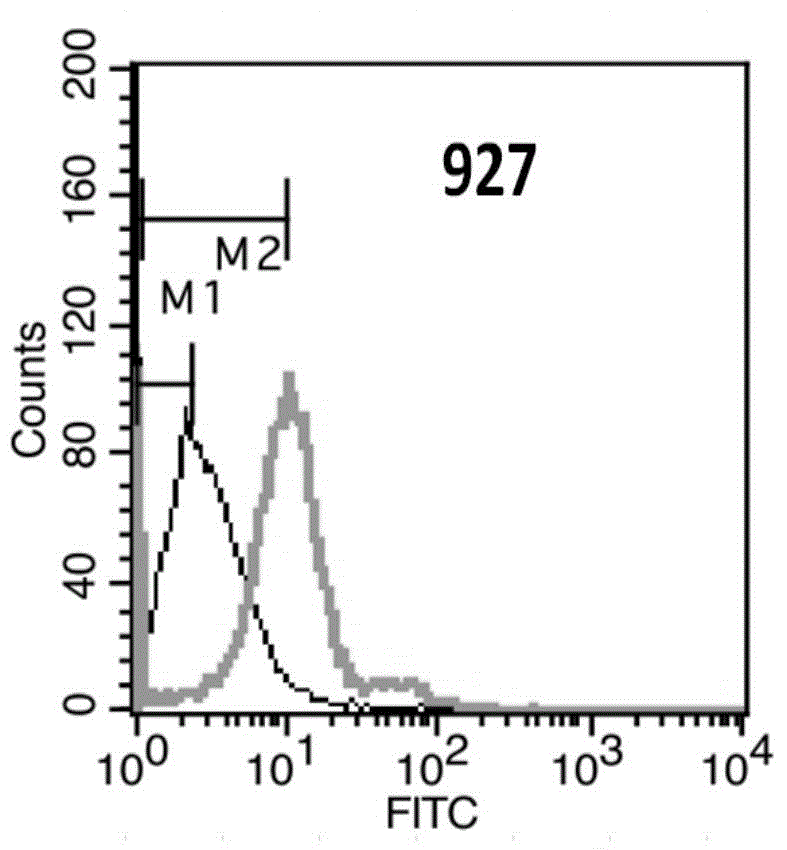
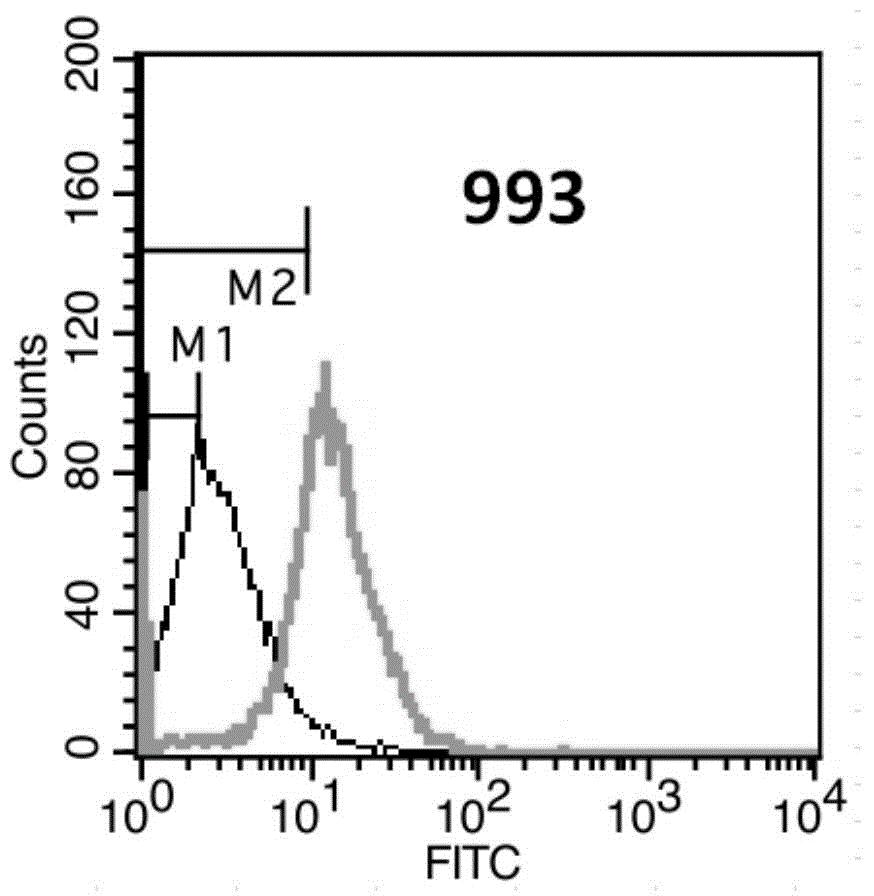
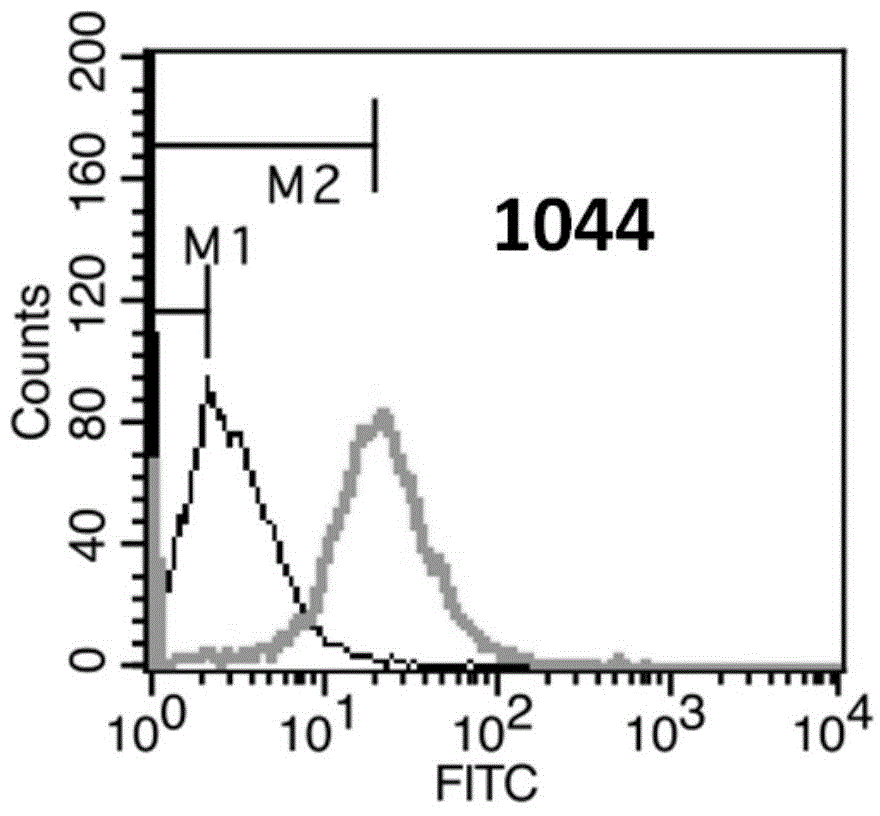
[0181] Example 3. Recognition of Her3 molecules on cell surface by anti-Her3 monoclonal antibody
[0182] Flow cytometry (FACS) was used for detection and indirect immunofluorescence staining.
[0183] (1) Counting: Take SKBR-3 cells in logarithmic growth phase, and adjust the concentration of single cell suspension to 2×106 / ml.
[0184] (2) Washing: take 1ml of single cell suspension and add it into a 1.5ml centrifuge tube, 1000rpm×3min. Discard the supernatant, wash and resuspend the cells with 2% PBA (PBS plus 2% fetal bovine serum), 1000 rpm×3min, discard the supernatant.
[0185] (3) Primary antibody incubation: dilute the anti-Her2 monoclonal antibody to 10 μg / ml with 2% PBA, add 200 μl, mix the cells by gently pipetting, and place in an ice bath at 4°C for 30 minutes. At the same time, a blank control and a mouse IgG monoclonal antibody isotype control were made. 1000rpm×3min, discard the supernatant.
[0186] (4) Fluorescent secondary antibody incubation: wash on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com