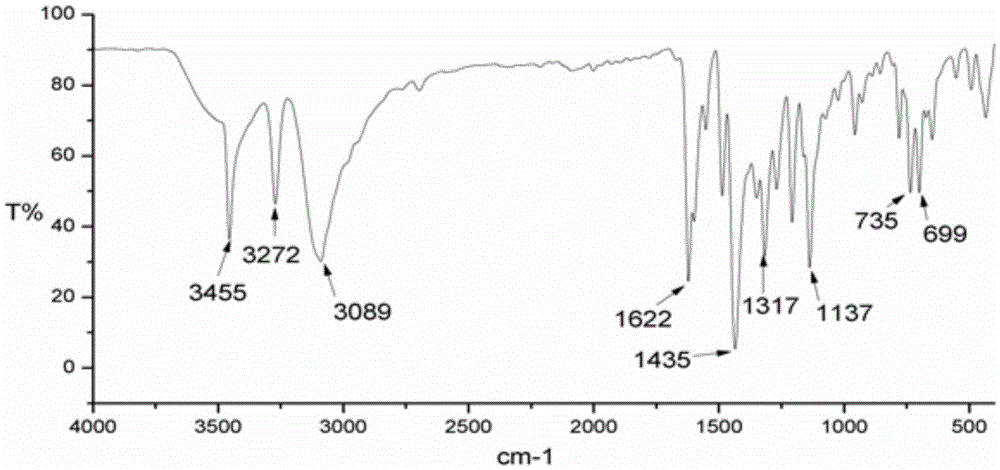
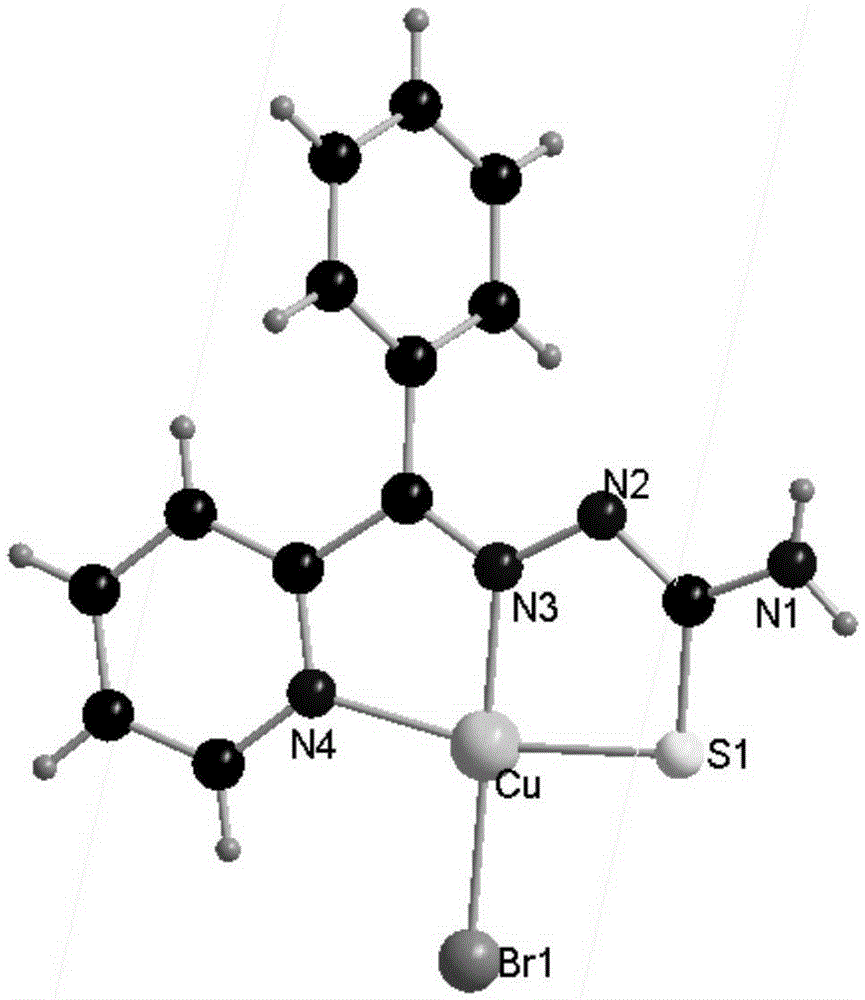
Human serum albumin-medicine compound, synthetic method and applications
A technology of human serum albumin and drug complexes, which is applied in the field of medicine to achieve the effects of low toxicity, improved targeting, and improved therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Synthesis of BpT complex
[0044] a) Dissolve 10mmol of 2-benzoylpyridine in 20ml of ethanol (the concentration of solvent ethanol is 50v / v%), stir for 15min at 60℃ to prepare a solution, drop the above solution into 20ml with 10mmol In a solution of thiosemicarbazide in ethanol (the concentration of solvent ethanol is 50v / v%), the reaction was refluxed and stirred at 60°C for 24 hours to obtain a pale yellow precipitate. The obtained pale yellow precipitate was filtered and washed with absolute ethanol and ether 3 times each After drying, the ligand 2-benzoylpyridine thiosemicarbazone is obtained;
[0045] b) Will contain CuBr 2 (223.35mg, 1mmol) 20ml of methanol (the concentration of solvent methanol is 60v / v%), added dropwise to 20ml of ethanol containing 1mmol of 2-benzoylpyridine thiosemicarbazone ligand (the concentration of solvent ethanol is 50v / v) %) in the solution, reflux and stir at 60°C for 2h, filter the reacted solution into a 50ml beaker and seal ...
Embodiment 2
[0051] Example 2: Synthesis of BpT complex
[0052] a) Dissolve 10mmol of 2-benzoylpyridine in 10ml of methanol (the concentration of solvent methanol is 80v / v%), and stir for 15min at 50℃ to prepare a solution. Drop the above solution into 20ml with 10mmol The thiosemicarbazide in ethanol (the concentration of solvent ethanol is 20v / v%) was refluxed and stirred at 80°C for 18h to obtain a pale yellow precipitate. The obtained pale yellow precipitate was filtered and washed with water 3 times. After drying, the preparation was obtained. Body 2-benzoylpyridine thiosemicarbazone;
[0053] b) Will contain CuBr 2 (223.35mg, 1mmol) 20ml of ethanol (solvent ethanol concentration is 40v / v%) solution, added dropwise to 20ml ethanol containing 1mmol 2-benzoylpyridine thiosemicarbazone ligand (solvent ethanol concentration is 70v / v %) in the solution, reflux and stir at 50°C for 2h, filter the reacted solution into a 50ml beaker and seal it with plastic wrap, pierce 30 holes at 8°C and vola...
Embodiment 3
[0054] Example 3: Synthesis of BpT complex
[0055] a) Dissolve 10mmol of 2-benzoylpyridine in 10ml of methanol (the concentration of solvent methanol is 80v / v%), and stir for 15min at 50℃ to prepare a solution. Drop the above solution into 20ml with 10mmol In a solution of thiosemicarbazide in methanol (solvent methanol concentration is 60v / v%), stir for 48h at 35°C to obtain a pale yellow precipitate. The obtained pale yellow precipitate is filtered and washed with methanol 3 times. After drying, Obtain the ligand 2-benzoylpyridine thiosemicarbazone;
[0056] b) Will contain CuBr 2 (223.35mg, 1mmol) 20ml of ethanol (solvent ethanol concentration is 30v / v%) solution, added dropwise to 20ml ethanol containing 1mmol 2-benzoylpyridine thiosemicarbazone ligand (solvent ethanol concentration is 20v / v %) in the solution, stirred at 40°C for 36h, filtered the reaction solution into a 50ml beaker and sealed with plastic wrap, needled 20 holes at 2°C and volatilized for several days to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com