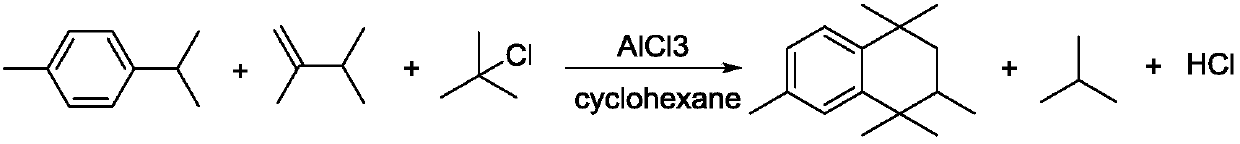
A continuous synthesis device and method for 1,1,3,4,4,6-hexamethyl-1,2,3,4-tetralin
A technology of tetralin and hexamethyl, applied in the direction of organic chemistry, addition of unsaturated hydrocarbons and saturated hydrocarbons to produce hydrocarbons, etc., can solve the problems of poor heat and mass transfer effect, low production capacity, etc., to improve production capacity and degree of automation High, improve the effect of utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
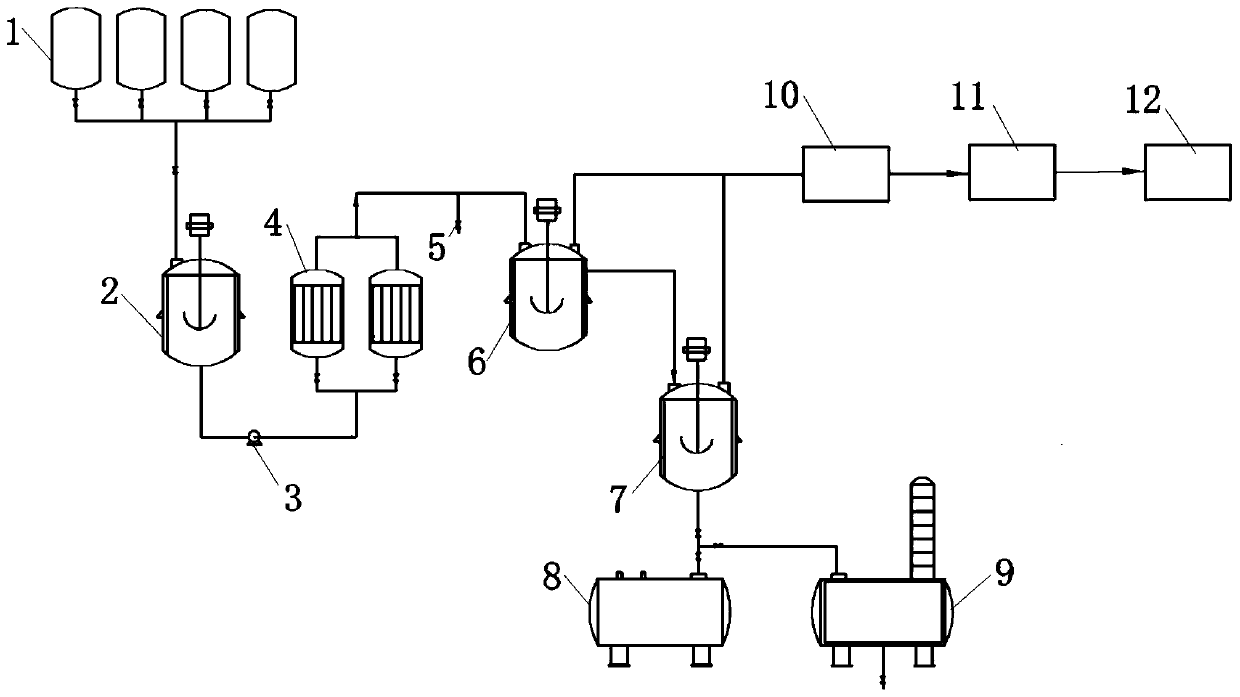
[0027] A method for the continuous synthesis of 1,1,3,4,4,6-hexamethyl-1,2,3,4-tetralin, comprising the following steps:
[0028] (Ⅰ) Raw material liquid mixing stage: Put the reaction raw materials paracymene, 2,3-dimethyl-1-butene and chloro-tert-butane and cyclohexane into the raw material storage tank respectively, and open the valve to enter the mixing The kettle is evenly mixed;
[0029] (II) Mixed liquid reaction stage: pump the mixture into the tubular reactor, contact with the aluminum trichloride catalyst filled in the reactor, and the Friedel-Crafts alkylation reaction occurs under the action of the catalyst, and the reaction liquid flows out of the tubular reactor , Flow into the transfer kettle, the transfer kettle further stirs the reaction liquid evenly by stirring, and collects the reaction liquid into the washing kettle by overflow method;
[0030] (Ⅲ) Post-treatment stage: add water to the water-washing kettle for quenching, then wash with alkali, and carry out rec...
Embodiment 1
[0036] Add 804kg of p-cymene, 252kg of 2,3-dimethyl-1-butene, 416kg of chloro-tert-butane and 1260kg of cyclohexane into the mixing kettle and stir for 0.5-1h. During the stirring process, 80 kg of aluminum trichloride was charged into the tubular reactor. After the filling is completed, adjust the temperature of the tubular reactor to 10-15°C, and start feeding through the centrifugal pump, and the feeding rate is controlled at 0.7-2.5 mL / s. The temperature in the transfer kettle is controlled at 10-30°C, and the stirring is started when the reaction liquid flows into the transfer kettle. After 4-6 hours of reaction, start sampling through the monitoring port every 30 minutes for monitoring, and replace the catalyst after the 1,1,3,4,4,6-hexamethyl-1,2,3,4-tetralin content decreases. The reaction liquid flows out through the overflow port. When the amount of reaction liquid in the washing kettle reaches 1800L, start adding 700L water to quench the reaction, and then wash with...
Embodiment 2
[0038] Add 402kg of p-cymene, 168kg of 2,3-dimethyl-1-butene, 222kg of chloro-tert-butane and 1008kg of cyclohexane into the mixing kettle and stir for 0.5-1h. During the stirring process, 60 kg of aluminum trichloride was charged into the tubular reactor. After the filling is completed, adjust the temperature of the tubular reactor to 15-20°C, and start feeding through the centrifugal pump, and the feeding speed is controlled at 2-4.5 mL s. The temperature in the transfer kettle is controlled at 15-20°C, and the stirring is started when the reaction liquid flows into the transfer kettle. After 3-5 hours of reaction, start sampling through the monitoring port every 30 minutes for monitoring, and replace the catalyst when the content of 1,1,3,4,4,6-hexamethyl-1,2,3,4-tetralin decreases. The reaction liquid flows out through the overflow port. When the amount of reaction liquid in the washing kettle reaches 1800L, start adding 700L water to quench the reaction, and then wash wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com