Anticancer composition and use thereof
A technology of composition and use, applied in the field of preparing medicine for treating cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
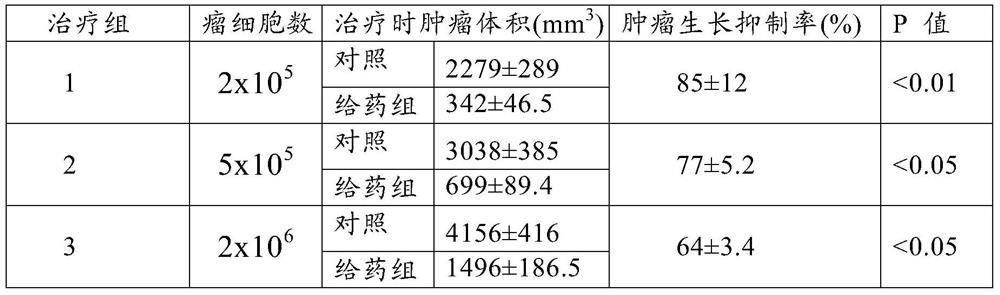
[0056] Example 1 Effects of Different Doses of Collagenase on the Growth of Rectal Tumors
[0057] Will 2x10 5A rectal cancer cell was subcutaneously injected into the quarter rib of the mice, and after 7 days of tumor growth, they were divided into the following 10 groups (6 rats in each group, see Table 1). Group 1 was the control group, groups 2-4 were collagenase Ⅱ treatment groups, groups 5-7 were collagenase Ⅳ treatment groups, and groups 8-10 were MMP-7 treatment groups. The drug was injected into the tumor, and the dose was calculated in kilograms of body weight (mg / kg) (see Table 1 for the specific dose), administered once a day for a total of 3 times. The tumor volume was measured on the 15th day after treatment, and the treatment effects of each group were compared (see Table 1). The activity of collagenase II used was 342 U / mg, the activity of collagenase IV was 180 U / mg, and the activity of MMP-7 was 186 U / mg. The activity of MMP-8 (collagenase II) in other exa...
Embodiment 2
[0063] Example 2 Comparison of Inhibitory Effects of Collagenase II on Thyroid Cancer When Administered Through Different Routes
[0064] Will 8x10 5 Thyroid carcinoma cells were subcutaneously injected into the quarter ribs of mice. After 7 days of tumor growth, they were divided into the following 10 groups (6 rats in each group, see Table 2), and collagenase II was injected through different routes. Drug doses are calculated in kilograms of body weight (mg / kg). The administration methods were tail vein injection (IV), intratumoral injection (IT) and intraperitoneal injection (IP). Administration once a day, a total of 3 times. The tumor volume was measured on the 15th day after treatment, and the treatment effects of each group were compared (see Table 2).
[0065] Table 2
[0066] Group (n=6) receive treatment Tumor volume (mm 3 )
[0067] As shown in the results in Table 2, different routes of administration of collagenase II have significant difference...
Embodiment 3
[0068] Example 3 Comparison of Inhibitory Effects of Collagenase II on Liver Cancer When Administered Through Different Routes
[0069] 5x105 liver cancer cells were subcutaneously injected into the quarter ribs of mice, and after 7 days of tumor growth, they were divided into the following 10 groups (6 rats in each group, see Table 3), and collagenase II was injected through different routes. Group 1 is the control group, groups 2-4 are collagenase II intratumoral injection (IT) group, groups 5-7 are collagenase II peritumoral injection (PT) group, and groups 8-10 are collagenase II subcutaneous injection group. Injection (SC, 1-2 cm from tumor margin) group. The dosage of the medicine is calculated according to kilogram body weight (U / kg), and each milliliter injection contains 1000U collagenase II. Administration once a day, a total of 3 times. The tumor volume was measured on the 15th day after treatment, and the treatment effects of each group were compared (see Table 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com