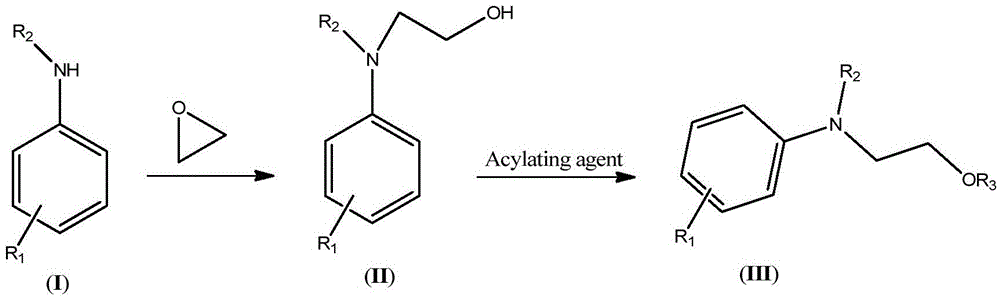
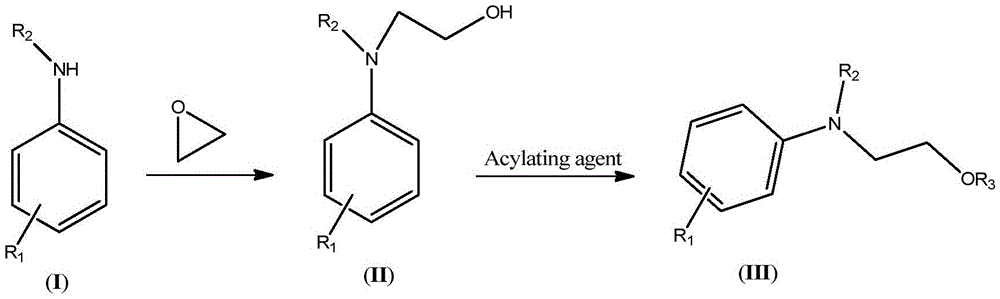
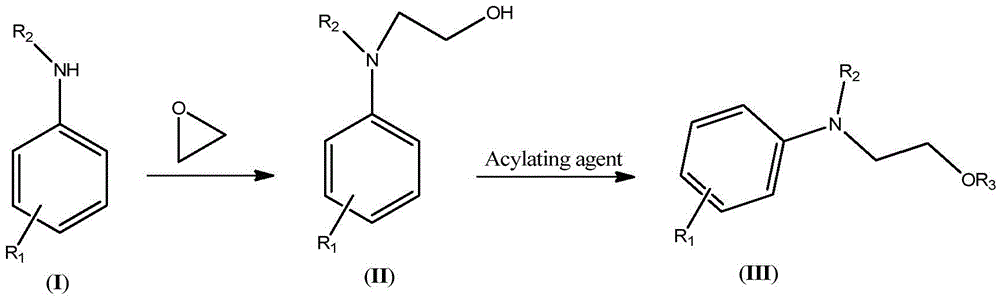
Synthetic technology of hydroxyethylaniline ester (III)
A technology of hydroxyethylaniline and dihydroxyethylaniline, which is applied in the field of synthesis technology of hydroxyethylaniline ester compound, can solve problems such as damage to society and people's livelihood, inflammability and explosion, hidden danger of ethylene oxide, etc., and achieve safety High performance, simple process, and the effect of increasing process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Synthesis of N,N-Dihydroxyethylaniline
[0043]Keep the temperature of the microreactor at 80-85°C, add 46.5g of aniline and 44g of ethylene oxide into the microreactor through syringe pumps-1 and -2 respectively, the flow rate of syringe pump-1 is 10mL / min, and the flow rate of syringe pump- The flow rate of 2 is 11mL / min. The reaction was completed to obtain 90.5 g (HPLC: 98.5%) of N,N-dihydroxyethylaniline. The NMR data of N,N-dihydroxyethylaniline are as follows: 1 HNMR (500MHz, CDCl 3 ): δ7.27(t, 2H), 6.94(d, 2H), 6.79(t, 1H), 4.20(t, 2H), 3.73(t, 2H), 3.65(br., 2H).
[0044] (2) Synthesis of N,N-diacetoxyethylaniline
[0045] Keep the microreactor temperature at 105-110°C, add 90.5g (HPLC: 98.5%) N,N-dihydroxyethylaniline and 105g acetic anhydride into the microreactor via syringe pumps -3 and -4, respectively, and the syringe pump The flow rates of -3 and -4 were both 10mL / min. After the reaction was complete, 135.2 g (HPLC: 99%) of crude N,N-diacetoxye...
Embodiment 2
[0047] (1) Synthesis of 2-methoxy-4-acetylamino-N,N-dihydroxyethylaniline
[0048] The temperature of the microreactor was kept at 90-100°C, and 720g of 2-methoxyl-5-acetamidoaniline aqueous solution (made of 180g 2-methoxyl-5-acetamidoaniline and 540g water Composition) and 89g of ethylene oxide were added to the microreactor, the syringe pump-1 was 36mL / min, and the syringe pump-2 was 5mL / min. The reaction was completed to obtain 266.6 g (HPLC: 98%) of 2-methoxy-4-acetamido-N,N-dihydroxyethylaniline. The NMR data of the 2-methoxy-4-acetylamino-N, N-dihydroxyethylaniline are as follows: 1 HNMR (500MHz, CDCl 3 ): δ7.27(br.,1H), 6.94(s,1H), 6.85(d,1H), 6.80(d,1H), 4.21(t,2H), 3.85(s,3H), 3.73(t ,2H), 3.65(br.,2H), 2.02(s,3H).
[0049] (2) Synthesis of 2-methoxy-4-acetylamino-N,N-diacetoxyethylaniline
[0050] Keep the temperature of the microreactor at 105-110°C, and inject 266.6g (HPLC: 98%) of 2-methoxy-4-acetamido-N,N-dihydroxyethylaniline through syringe pumps -3 and -...
Embodiment 3
[0052] (1) Synthesis of 3-methyl-4-(2,2-dicyanovinyl)-N-ethyl-N-hydroxyethylaniline
[0053] Keep the temperature of the microreactor at 90-100°C, and inject 1266g of 3-methyl-4-(2,2-dicyanovinyl)-N-ethylaniline aqueous solution [from 211g3 -Methyl-4-(2,2-dicyanovinyl)-N-ethylaniline and 1055g of water] and 48.4g of ethylene oxide were added to the microreactor, the syringe pump-1 was 50mL / min, and the injection Pump-2 was 1.9 mL / min. The reaction was completed to obtain 257.6 g (HPLC: 96.6%) of 3-methyl-4-(2,2-dicyanovinyl)-N-ethyl-N-hydroxyethylaniline. The 3-methyl-4-(2,2-dicyanovinyl)-N-ethyl-N-hydroxyethylaniline such as: 1 HNMR (500MHz, CDCl 3 ): δ8.06(s,1H), 7.10(d,1H), 6.63(s,1H), 6.52(d,1H), 4.24(t,2H), 3.75(t,2H), 3.66(br. ,1H), 3.41(q,2H), 2.50(s,3H), 1.16(t,3H).
[0054] (2) Synthesis of 3-methyl-4-(2,2-dicyanovinyl)-N-ethyl-N-benzoyloxyethylaniline
[0055] Keep the temperature of the microreactor at 105-110°C, and inject 257.6g (HPLC: 96.6%) of 2-methoxy-4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com