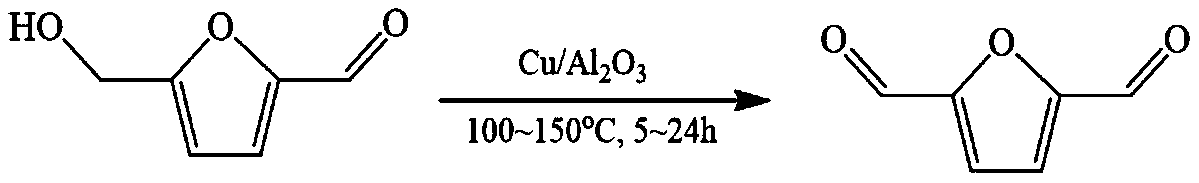Method for preparing 2,5-difuralehyde from 5-hydroxymethyl furfural through dehydrogenation
A technology of hydroxymethylfurfural and furandicarbaldehyde, applied in the field of 5-hydroxymethylfurfural dehydrogenation to prepare 2,5-furandicarbaldehyde, which can solve the problem of flammability and explosion of molecular oxygen, low atom utilization rate and low catalytic efficiency and other problems, to achieve the effect of high atom utilization, good application prospects, and good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of described catalyst is also known technology, and the following is a specific example (but not limited thereto):
[0029] The first step is to measure the saturated water absorption of the carrier: accurately weigh 1.0g of each carrier on a watch glass, add pure water drop by drop until the carrier is completely wet and slightly flowing, and record the volume of pure water used as the saturated water absorption of the carrier .
[0030] The second step, preparing the supported metal catalyst by impregnation method: accurately weigh 2.0 g of the carrier and place it in a crucible. Accurately weigh 0.4g Cu(NO 3 ) 2 ·3H 2 O in a beaker, add the saturated water absorption of the carrier measured in the first step, and dissolve completely. Add the copper solution to the carrier drop by drop until the carrier is completely wetted and slightly flowing. After standing for 12 hours, the catalyst was calcined in a muffle furnace at 400° C. for 4 hour...
Embodiment 1
[0033] 5-Hydroxymethylfurfural (5mmol), mesitylene (15mL) and reduced 6.6wt% Cu / γ-Al 2 o 3 Catalyst (1.0g) adds in the autoclave, feeds N 2 The air in the reaction kettle was evacuated, and the reaction was stopped after mechanical stirring at 150° C. for 12 hours. The reaction was cooled to room temperature, and the supernatant was separated by centrifugation. The catalyst sank to the bottom, and the supernatant was taken for direct analysis on gas chromatography. The result of the reaction was that the conversion rate of 5-hydroxymethylfurfural was 58.0%. The yield of formaldehyde was 36.6%.
[0034] Among them, the detection method of the product: after the reaction is finished, the reaction solution is centrifuged, the upper layer is a colored clear liquid, and the lower layer is a metal catalyst. The supernatant was centrifuged, filtered, and analyzed by gas chromatography.
Embodiment 2
[0036] Other steps are the same as in Example 1, except that the catalyst added is non-reducing 6.6wt% Cu / γ-Al 2 o 3 , The reaction result is that the conversion rate of 5-hydroxymethylfurfural is 44.9%, and the yield of 2,5-furandicarbaldehyde is 35.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com