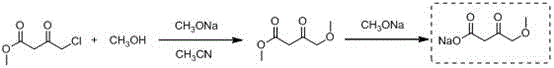
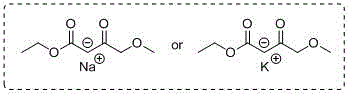
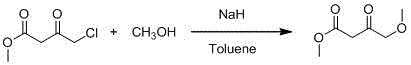Synthetic method of 4-methoxyethyl acetoacetate
A technology of ethyl methoxyacetoacetate and ethyl chloroacetoacetate, which is applied in the field of synthesis of ethyl 4-methoxyacetoacetate, can solve the problems of unaffordable and expensive molecular distillation equipment, and achieve simple and effective removal The effect of removing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 250mL tetrahydrofuran to the 2L reaction bottle in advance, and start stirring under the protection of argon. At this time, the internal temperature is 25°C, and 55g (1.35mol) sodium hydride (containing 40% mineral After the addition, continue to add 450 mL of tetrahydrofuran; slowly add a mixed solution of 30 g of methanol and 100 g (0.61 mol) of ethyl 4-chloroacetoacetate dropwise at an internal temperature of 20 ° C, and adjust the stirring speed at about 310 rpm , the addition was completed in about 4 hours; the internal temperature rose to 20-25°C and stirred for 5 hours, and the reaction was detected by TLC; the system began to cool down, and it could be seen that the color of the solution was light yellow and a large amount of solids were suspended. , when the internal temperature drops to -5°C, slowly add 100mL of hydrochloric acid solution with a molar concentration of 2mol / L, keep the internal temperature below -2°C, and finish adding in about 20 minutes. A...
Embodiment 2
[0025] Add 250mL tetrahydrofuran to the 2L reaction bottle in advance, and start stirring under the protection of argon. At this time, the internal temperature is 25°C, and 55g (1.35mol) sodium hydride (containing 40% mineral After the addition, continue to add 450 mL of tetrahydrofuran; slowly add a mixed solution of 30 g of methanol and 100 g (0.61 mol) of ethyl 4-chloroacetoacetate dropwise at an internal temperature of 20 ° C, and adjust the stirring speed at about 310 rpm , the addition was completed in about 4 hours; the internal temperature rose to 20-25°C and stirred for 5 hours, and the reaction was detected by TLC; the system began to cool down, and it could be seen that the color of the solution was light yellow and a large amount of solids were suspended. , when the internal temperature drops to -5°C, slowly add 100mL of hydrochloric acid solution with a molar concentration of 2mol / L, keep the internal temperature below -2°C, and finish adding in about 20 minutes. A...
Embodiment 3
[0027]Add 250mL tetrahydrofuran to the 2L reaction bottle in advance, and start stirring under the protection of argon. At this time, the internal temperature is 25°C, and 55g (1.35mol) sodium hydride (containing 40% mineral After the addition, continue to add 450 mL of tetrahydrofuran; slowly add a mixed solution of 30 g of methanol and 100 g (0.61 mol) of ethyl 4-chloroacetoacetate dropwise at an internal temperature of 20 ° C, and adjust the stirring speed at about 310 rpm , the addition was completed in about 4 hours; the internal temperature rose to 20-25°C and stirred for 5 hours, and the reaction was detected by TLC; the system began to cool down, and it could be seen that the color of the solution was light yellow and a large amount of solids were suspended. , when the internal temperature drops to -7°C, slowly add 100mL of hydrochloric acid solution with a molar concentration of 2mol / L, keep the internal temperature below 0°C, and finish adding in about 20 minutes. At ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com