Natural antiepileptic active compounds and their use in pharmaceutical preparations
An anti-epileptic and compound technology, applied in the field of natural anti-epileptic active compounds and their pharmaceutical preparations, can solve problems such as difficulty in quality control, unknown targets, and complex components of Qingyang ginseng tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
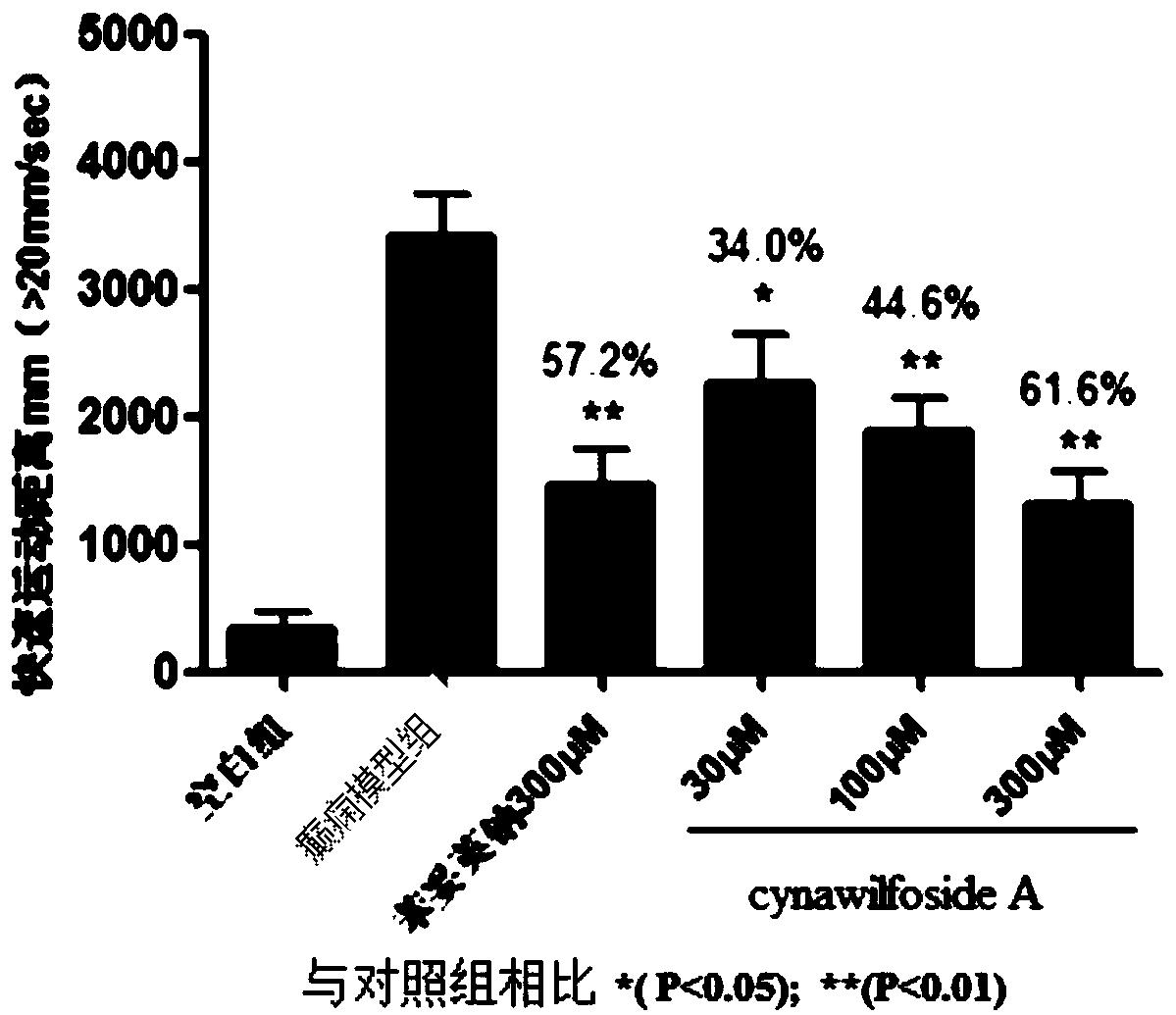
[0170] Evaluation of antiepileptic effect of compound cynawilfoside A (formula VI) in vivo:
[0171] The preparation method of the compound cynawilfoside A: 10kg of fresh Geshanxiao roots, peeled and sliced to obtain 2.7kg of dry product, crushed and extracted three times with 95% ethanol percolation, each time for 2-3 days, combined extracts, concentrated under reduced pressure to obtain Geshan Disinfected alcoholic extract, suspend the extract in water, extract with ethyl acetate to obtain ethyl acetate extract, mix the sample with 100 mesh silica gel, separate with silica gel column chromatography (200-300 mesh silica gel), and use chloroform- Gradient elution with methanol (10:1→5:1) yielded components Fr1-Fr9. Fr6 was further purified repeatedly with petroleum ether-acetone and preparative HPLC to obtain pure compound cynawilfoside A (1.4 g).
[0172]
[0173] The physical and chemical data of the compound cynawilfoside A are as follows: white amorphous powder, easi...
Embodiment 2
[0178] Evaluation of antiepileptic effect of compound wilfoside C1N (formula IV) in vivo:
[0179] The preparation method of Wilfoside C1N: a. 10kg of fresh Auricularia saponis root tuber, sliced and dried to obtain 3Kg dry product, pulverized and extracted three times with 95% ethanol, the extracts were combined, concentrated under reduced pressure to obtain ethanol extract, mixed with silica gel, passed 200-300 mesh silica gel, gradient elution with petroleum ether-acetone (3:1→2:1), and then gradient elution with chloroform-methanol (10:1→5:1) to obtain components Fr1-Fr6. Fr3 was further purified repeatedly by petroleum ether-acetone silica gel column chromatography to obtain pure compound wilfoside C1N (1.5g); b. 10kg of fresh Geshanxiao root, peeled, sliced and dried to obtain 2.7kg dry product, pulverized and diafiltered with 95% ethanol Extract three times, each time for 2-3 days, combine the extracts, concentrate under reduced pressure to obtain the alcohol extrac...
Embodiment 3
[0185] Evaluation of compound wilfoside K1N (formula V) antiepileptic effect in vivo:
[0186] The preparation method of Wilfoside K1N: a. 10kg of fresh cowhide tuber, sliced and dried to obtain 3kg of dry product, pulverized and extracted three times with 95% ethanol, the extracts were combined, concentrated under reduced pressure to obtain ethanol extract, mixed with silica gel, passed 200- 300 mesh silica gel, gradient elution with petroleum ether-acetone (3:1→2:1), and then gradient elution with chloroform-methanol (10:1→5:1) to obtain fractions Fr1-Fr6. Fr3 was further purified repeatedly by petroleum ether-acetone silica gel column chromatography to obtain pure compound wilfoside K1N (2.5g); b. 10kg of fresh Geshanxiao root, peeled and sliced to obtain 2.7kg of dry product, crushed and percolated with 95% ethanol Extract three times, each time for 2-3 days, combine the extracts, concentrate under reduced pressure to obtain the alcohol extract of Geshanxiao, suspend t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com