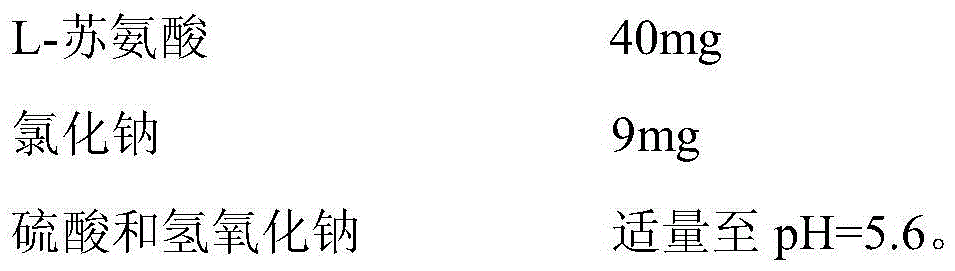Tobramycin inhalation solution and preparing method
A technology of tobramycin and solution, applied in the field of medicine, can solve the problems of limited formation, aggravation of adverse reactions, induction of lung disease patients, etc., and achieve the effect of reducing the incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Tobramycin inhalation solution of the present invention, every 2ml (1 unit dose vial) contains:
[0028]
[0029]
[0030] The preparation method of above-mentioned tobramycin inhalation solution, the steps are as follows:
[0031] Dissolve 9g of sodium chloride in purified water, stir to fully dissolve, use 98.3wt% concentrated sulfuric acid to adjust the pH, then add 150g tobramycin and 40gL-threonine, and mix until completely dissolved, use 98.3wt% concentrated sulfuric acid Sulfuric acid or 1mol / L sodium hydroxide solution to adjust the pH, and then continue to add purified water to 2L. Finally, it is sufficient to filter the obtained aqueous solution through 0.45 μm and 0.2 μm nylon filters in sequence. The solution was filled into 2 ml polyethylene colorless unit dose vials under a nitrogen purge.
Embodiment 2
[0033] Tobramycin inhalation solution of the present invention, every 2ml (1 unit dose vial) contains:
[0034]
[0035] The preparation method of above-mentioned tobramycin inhalation solution, the steps are as follows:
[0036] Dissolve 9g of sodium chloride in purified water, stir to fully dissolve, adjust pH with 98.3wt% concentrated sulfuric acid, then add 150g tobramycin and 60gL-threonine, and mix until completely dissolved, use 98.3wt% concentrated sulfuric acid Sulfuric acid or 1mol / L sodium hydroxide solution to adjust the pH, and then continue to add purified water to 2L. Finally, it is sufficient to filter the obtained aqueous solution through 0.45 μm and 0.2 μm nylon filters in sequence. The solution was filled into 2 ml polyethylene colorless unit dose vials under a nitrogen purge.
Embodiment 3
[0038] Tobramycin inhalation solution of the present invention, every 2ml (1 unit dose vial) contains:
[0039]
[0040] The preparation method of above-mentioned tobramycin inhalation solution, the steps are as follows:
[0041] Dissolve 9g of sodium chloride in purified water, stir to fully dissolve, adjust pH with 98.3wt% concentrated sulfuric acid, then add 150g tobramycin and 50gL-threonine, and mix until completely dissolved, use 98.3wt% concentrated sulfuric acid Sulfuric acid or 1mol / L sodium hydroxide solution to adjust the pH, and then continue to add purified water to 2L. Finally, it is sufficient to filter the obtained aqueous solution through 0.45 μm and 0.2 μm nylon filters in sequence. The solution was filled into 2 ml polyethylene colorless unit dose vials under a nitrogen purge.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com