Use of edaravone in preparation of drug for preventing and treating cerebral amyloid angiopathy (CAA)
A technology for edaravone and drug preparation, which is applied in the pharmaceutical field to achieve the effects of preventing continued deposition, inhibiting Aβ aggregation, and eliminating Aβ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
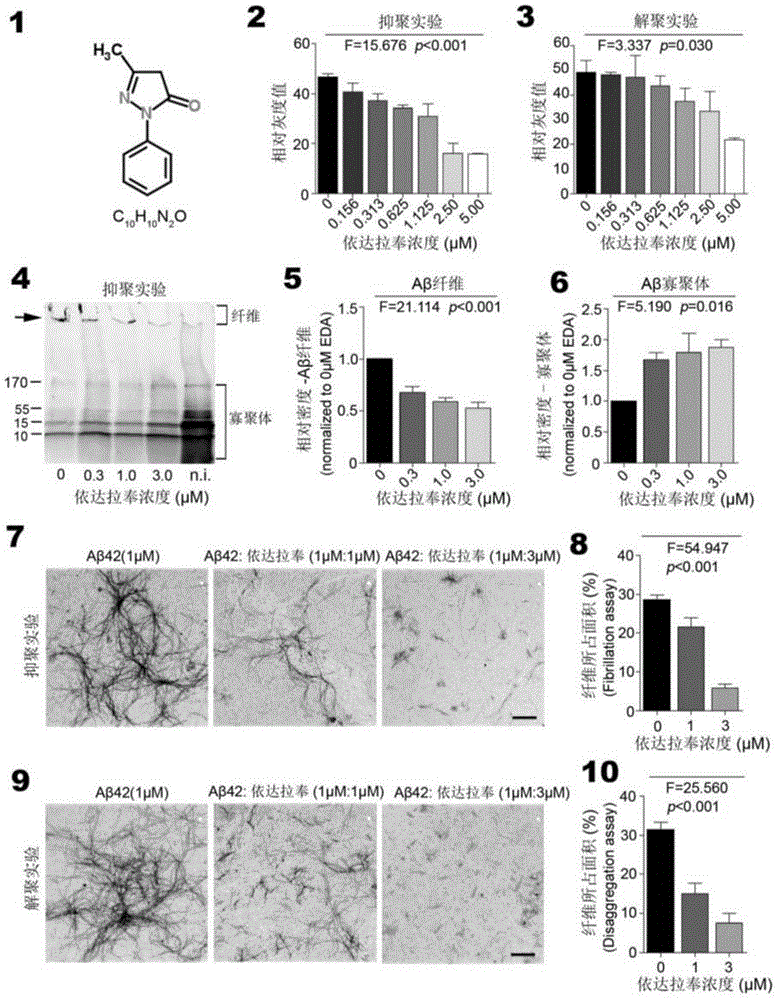
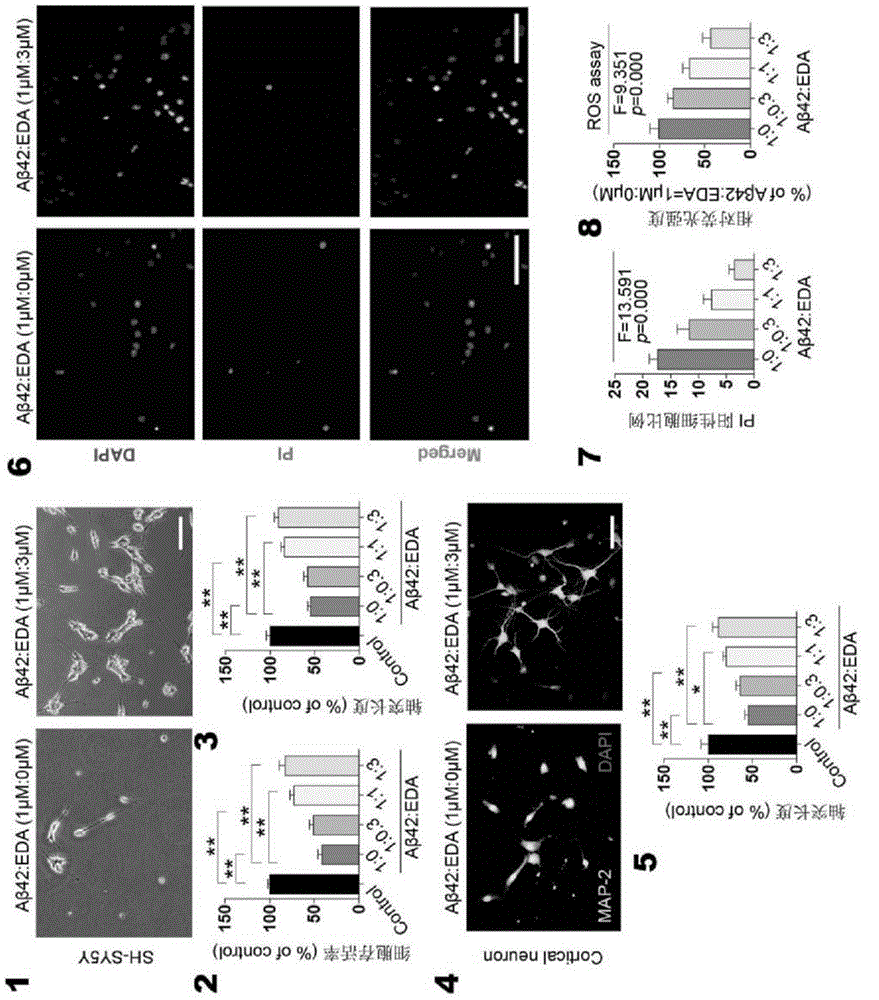
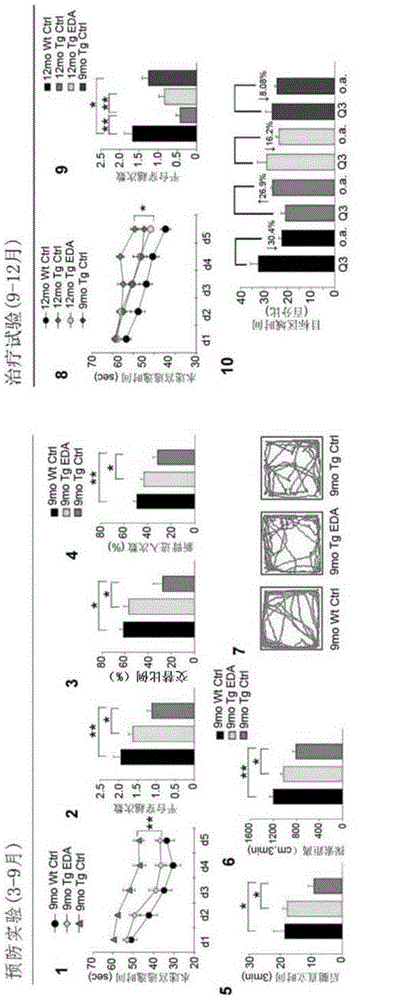
[0042] In order to further understand the present invention, preferred solutions of the present invention will be described below in conjunction with examples. These descriptions are only to illustrate the features and advantages of the technical solutions of the present invention, but not to limit the protection scope of the present invention.
[0043] 1. Thioflavin T fluorescence test
[0044] Inhibitory effect of Edaravone on Aβ aggregation: 10 μM Aβ42 and different concentrations of Edaravone solutions (0 μM, 1.56 μM, 3.13 μM, 6.25 μM, 11.25 μM, 25.0 μM, 50.0 μM and 100 μM) in a 37°C incubator Incubate for two days, add 5 μM Thioflavin T working solution and continue to incubate for 20 minutes, and read the OD value with a microplate reader (excitation wavelength is 450 nm, emission wavelength is 482 nm).
[0045] Depolymerization of Edaravone on Aβ fibers: Aβ42 was dissolved in DMEM medium and incubated at 37°C for two days to form Aβ fibers. The formed Aβ fibers were i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com