A method for identifying the optical activity of optical isomers
An optical isomerization and compound technology, applied in the direction of polarization effect characteristics, etc., can solve the problems of complicated and cumbersome use process, time-consuming and difficult operation of accessories, and achieve the effect of ensuring food and drug safety, promoting life science, and improving production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
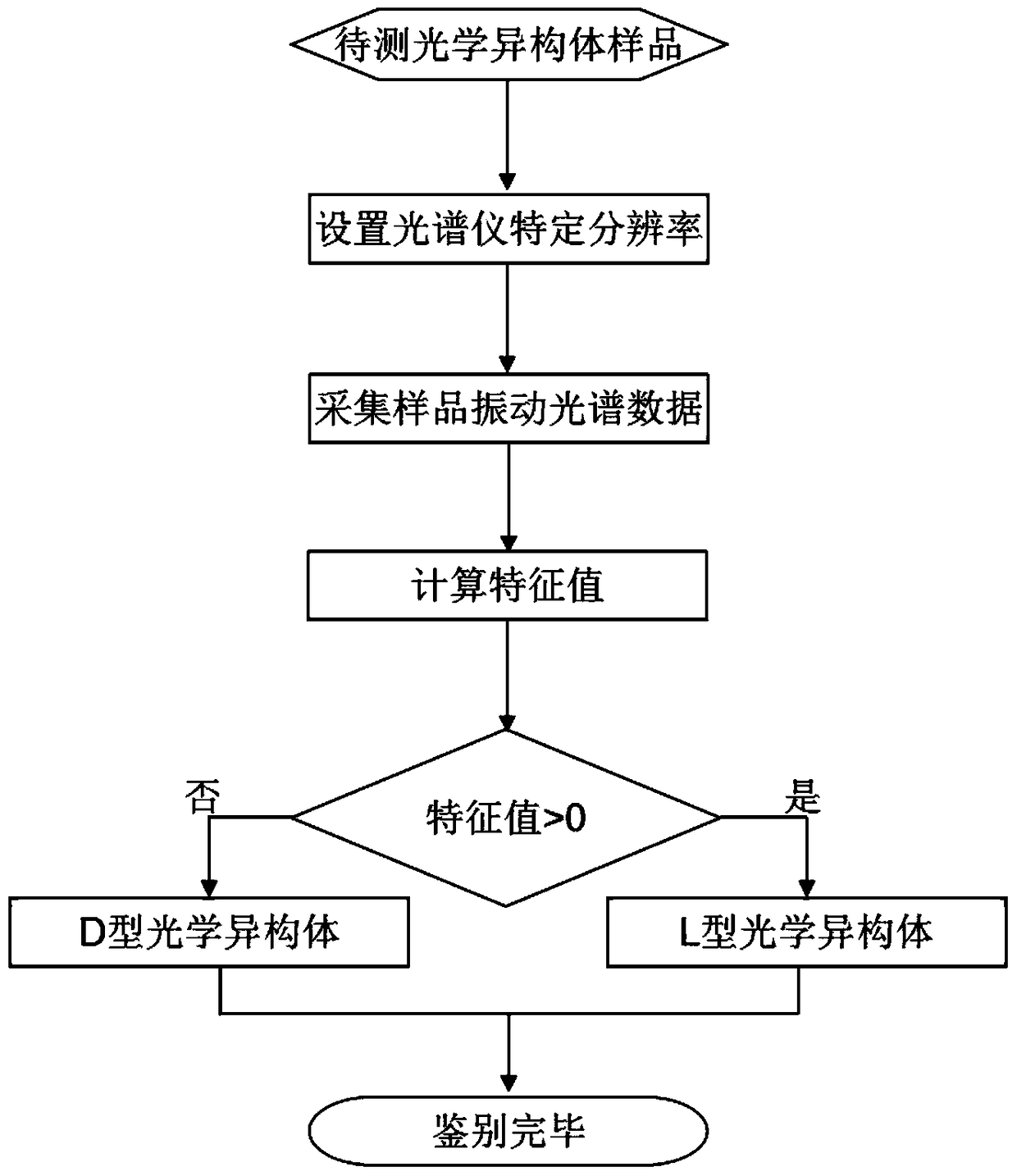
[0035] Follow the steps below to detect the optical activity of sample A to be tested:
[0036] (1) Set the resolution of the Fourier transform mid-infrared spectrometer to 4cm -1 ;
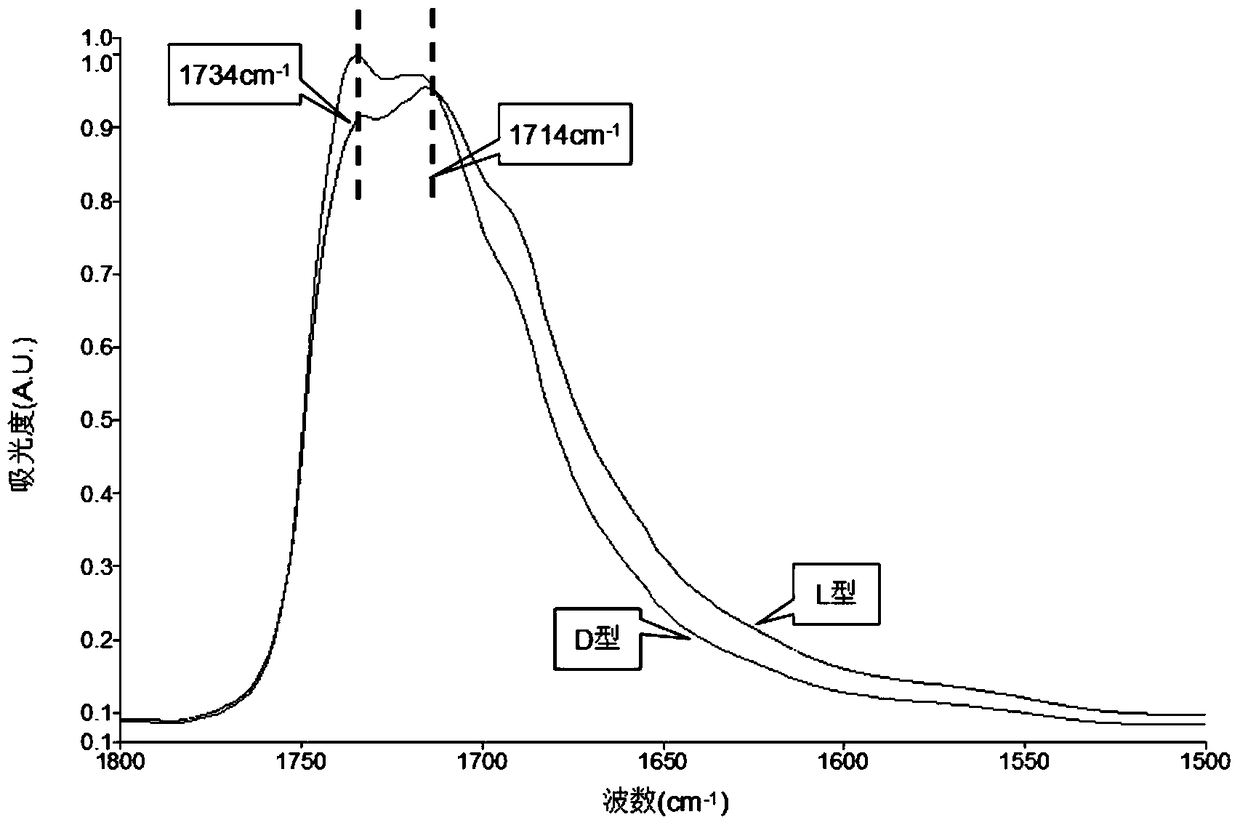
[0037] (2) At a resolution of 4cm -1 Under certain conditions, collect the ATR-MIR spectrum of the sample to be tested, and perform ATR correction to eliminate the refraction difference of mid-infrared light with different wavenumbers in the ATR crystal; the absorbance is used as the ordinate, and the wavenumber is used as the Abscissa, to obtain the sample to be tested in the spectral range of 1800 ~ 1500cm -1 The spectrogram inside (refer to image 3 shown);
[0038] from image 3 Read out the characteristic wavenumber of the sample to be tested in 1 =1734cm -1 Absorbance of 1 is 0.9155, at the characteristic wavenumber 2 =1714cm -1 Absorbance of 2 is 0.9543;
[0039] (3) Calculate the characteristic value of the sample to be tested according to the following formula;
[0040]
...
Embodiment 2
[0044] Adopt the method described in Example 1 to detect the optical rotation of the sample B to be tested, and the specific steps are:
[0045] (1) Set the resolution of the Fourier transform mid-infrared spectrometer to 4cm-1 ;
[0046] (2) At a resolution of 4cm -1 Under the condition, collect the mid-infrared spectrum absorption spectrum of the sample to be tested (refer to image 3 shown), to obtain samples at the characteristic wavenumber 1 =1734cm -1 Absorbance of 1 is 0.9985 and the characteristic wavenumber 2 =1714cm -1 Absorbance of 2 is 0.9601;
[0047] (3) Calculate the characteristic value of the sample to be tested according to the following formula;
[0048]
[0049] (4) After calculation, the characteristic value of the sample to be tested is -19.2<0, and it is judged that the sample to be tested is D-tartaric acid (D-tartaric acid).
[0050] Through the verification of the structure of the sample to be tested, the result of judging that it is D-tar...
Embodiment 3
[0053] In addition, 50 groups of L-tartaric acid and 50 groups of D-tartaric acid with known optical activity were collected according to the method described in Example 1 to collect attenuated total reflection-mid-infrared spectra, and processed according to the above method. The results show that the eigenvalues of L-tartaric acid are all greater than 0, the eigenvalues of D-tartaric acid are all less than 0, and the difference between the minimum value of L-tartaric acid and the maximum value of D-tartaric acid is greater than 30. The above results illustrate that the Fourier transform mid-infrared spectrometer adopted in this embodiment has a spectral resolution of 4cm -1 Under this method, L-tartaric acid and D-tartaric acid can be quickly and non-destructively identified without polarizing accessories.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com