Nasal spray and preparation method thereof
A production method and technology of nasal spray, applied in the direction of medical formula, aerosol delivery, medical preparations containing active ingredients, etc., can solve the problems of stimulating nasal secretions, excretion, etc., reduce the rate of volatilization loss, reduce the Good dosage and dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
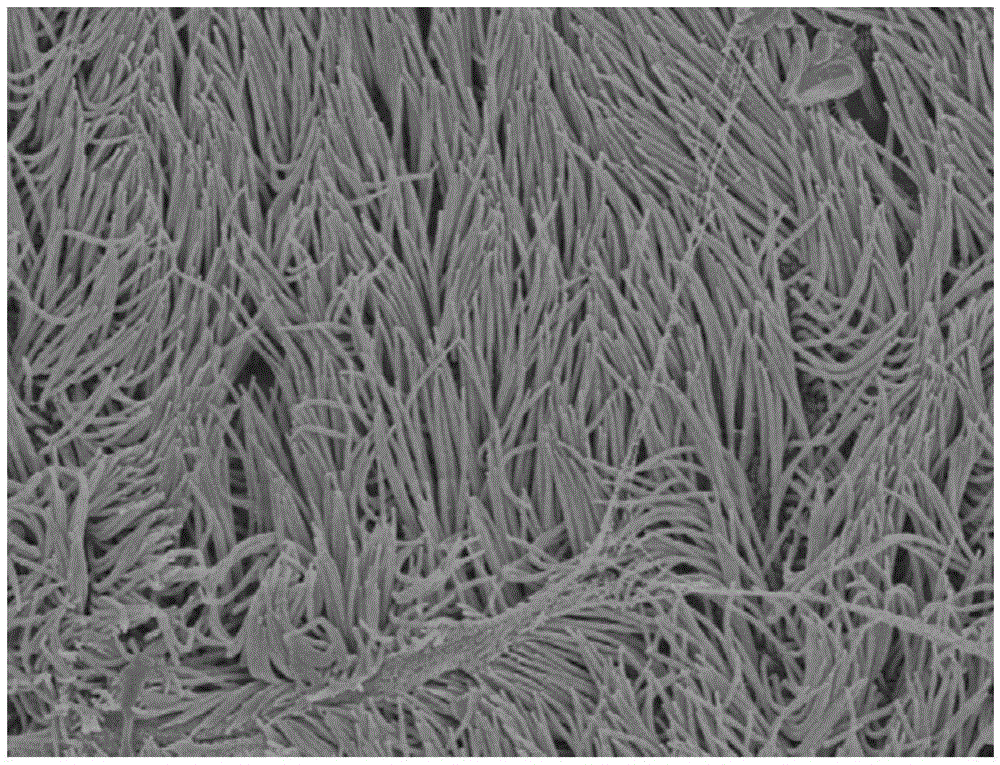
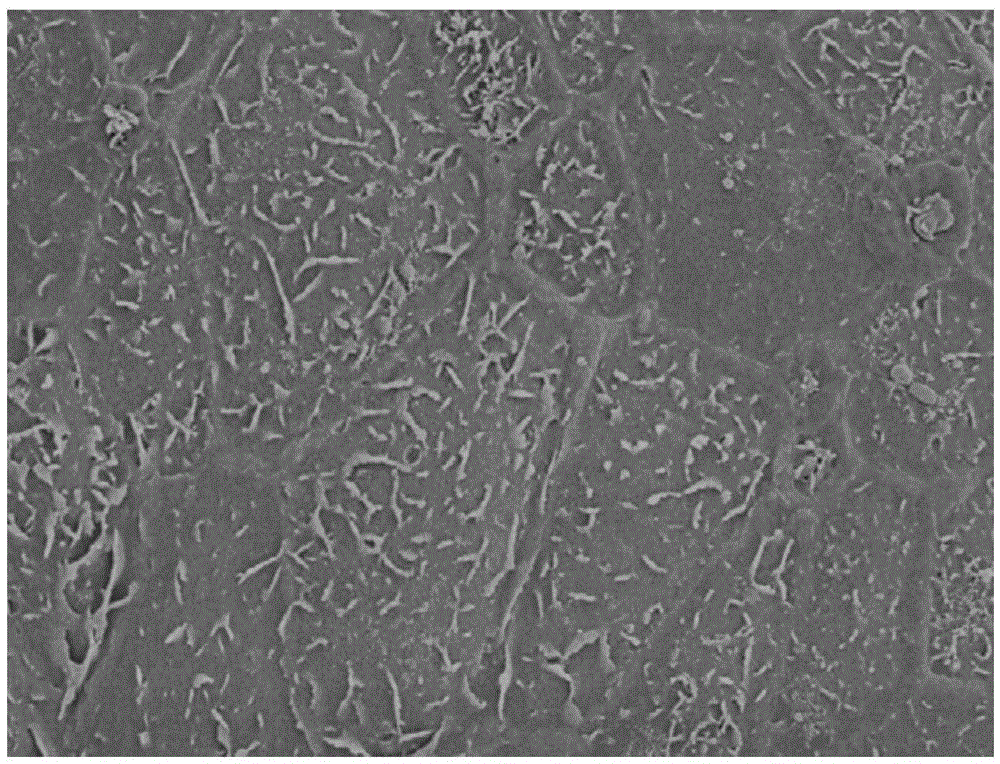
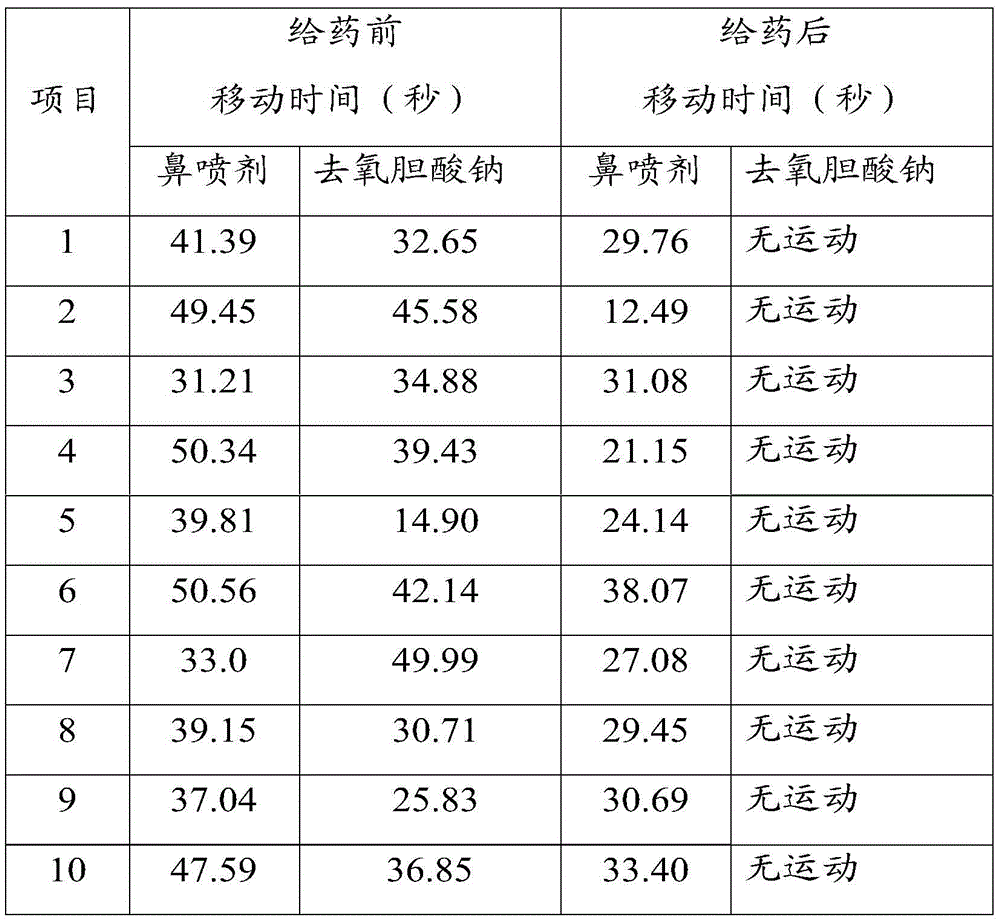
Image
Examples
Embodiment 1
[0041] This embodiment provides a nasal spray with a pH value of 6.6 and an osmotic pressure of 316 mmoL / L. The nasal spray includes polyoxyethylene hydrogenated castor oil, L-α-phosphatidylcholine lecithin, injection grade soybean oil, water, essential oil. The essential oil is composed of 1.8-cineole, d-limonene, and α-pinene, and the volume ratio of 1.8-cineole, d-limonene, and α-pinene is 8:30:45.
[0042] Above-mentioned nasal spray is made by following method:
[0043] Step 1: Add 0.6 g of polyoxyethylene hydrogenated castor oil into 100 ml of water, mix and stir to form an aqueous phase solution.
[0044] In the second step, 3 ml of essential oil, 0.3 g of L-α-phosphatidylcholine lecithin and 0.5 g of injection-grade soybean oil were stirred and mixed to obtain an oil phase solution.
[0045] In the third step, the oil phase solution is mixed with the water phase solution, and emulsified at a speed of 10,000 rpm by a high-speed shear emulsifier to obtain coarse milk. ...
Embodiment 2
[0049]This embodiment provides a nasal spray with a pH value of 6.6 and an osmotic pressure of 320 mmoL / L. The nasal spray includes polyoxyethylene hydrogenated castor oil, L-α-phosphatidylcholine lecithin, injection grade soybean oil, water, essential oil. The essential oil is composed of 1.8-cineole, d-limonene, and α-pinene, and the volume ratio of 1.8-cineole, d-limonene, and α-pinene is 12:30:50.
[0050] Above-mentioned nasal spray is made by following method:
[0051] Step 1: Add 1 gram of polyoxyethylene hydrogenated castor oil into 97 milliliters of water, mix and stir to form an aqueous phase solution.
[0052] In the second step, 2.5 ml of essential oil, 0.3 g of L-α-phosphatidylcholine lecithin and 2.4 ml of injection-grade soybean oil were stirred and mixed to obtain an oil phase solution.
[0053] In the third step, the oil phase solution is mixed with the water phase solution, and emulsified at a speed of 11,000 rpm by a high-speed shear emulsifier to obtain c...
Embodiment 3
[0056] This embodiment provides a nasal spray with a pH value of 6.7 and an osmotic pressure of 311 mmoL / L. The nasal spray includes polyoxyethylene hydrogenated castor oil, L-α-phosphatidylcholine lecithin, injection grade soybean oil, water, essential oil. The essential oil is composed of 1.8-cineole, d-limonene, and α-pinene, and the volume ratio of 1.8-cineole, d-limonene, and α-pinene is 12:36:47.
[0057] Above-mentioned nasal spray is made by following method:
[0058] Step 1: Add 3.5 grams of polyoxyethylene hydrogenated castor oil into 130 milliliters of water, mix and stir to form an aqueous phase solution.
[0059] In the second step, 6 mL of essential oil, 1.2 g of L-α-phosphatidylcholine lecithin and 7.8 mL of injection-grade soybean oil were stirred and mixed to obtain an oil phase solution.
[0060] In the third step, the oil phase solution and the water phase solution are mixed at a volume ratio of 8:15, emulsified with a high-pressure homogenizer at 2°C and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com