Preparation method of eliglustat
A compound and organic solvent technology, applied in the field of medicinal chemical synthesis, can solve the problems of low purity and difficult removal of the final product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
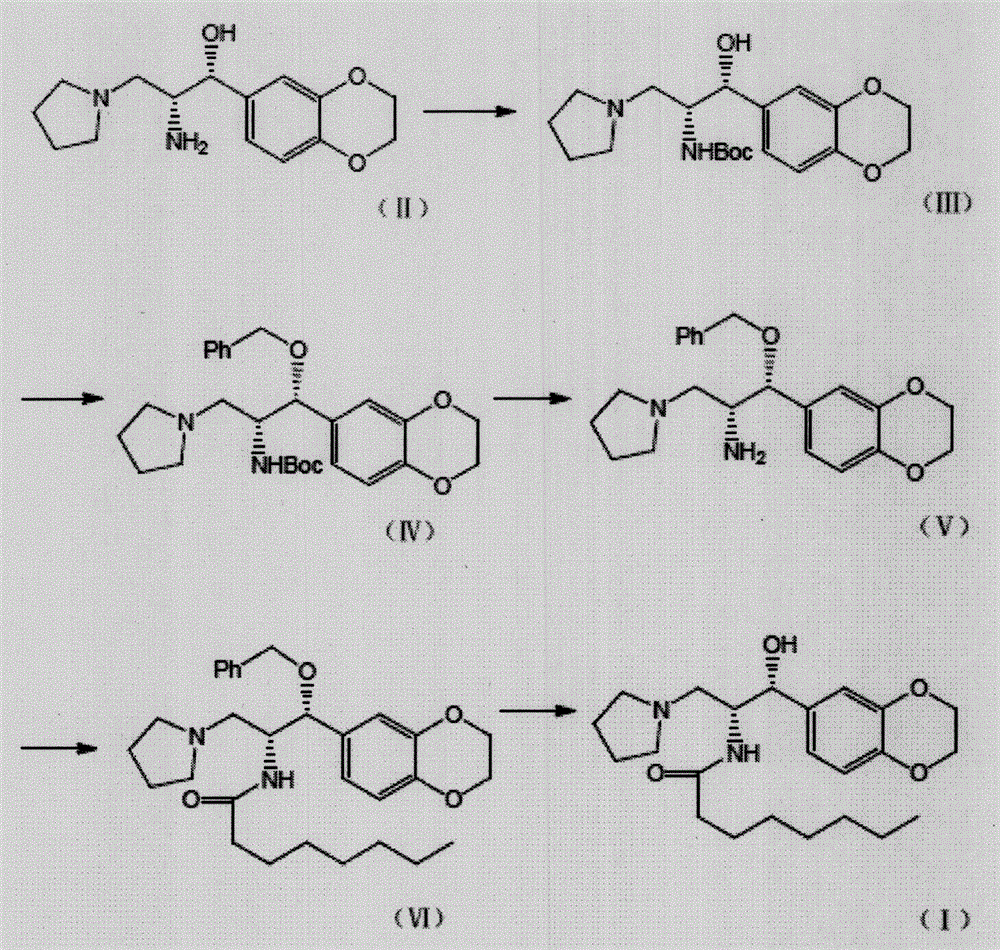
[0047] The preparation of embodiment 1 formula (III) compound
[0048] In a 5L round-bottomed three-neck flask equipped with a drying tube, add the compound of formula (II) (278g, 1mol) and acetonitrile (2.8L), add potassium carbonate (276g, 2mol) under stirring, and add Boc anhydride dropwise in an external bath at 10°C (327g, 1.5mol) in acetonitrile (300mL) solution, continue to react for 3h after addition, then add water (3L), extract three times with ethyl acetate (800mL×3), combine organic phases, wash with saturated brine (800mL) washed, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 368 g of light yellow oil, with a yield of 97.6%.
Embodiment 2
[0049] The preparation of embodiment 2 formula (IV) compound
[0050]In a 5L round-bottomed three-neck flask equipped with a drying tube, add the compound of formula (III) (302g, 0.8mol) and DMF (3.6L), after stirring and dissolving, add potassium carbonate (276g, 2mol) and benzyl bromide (205g , 1.2mol), continued to react for 7h in an external bath at 160°C, cooled, poured into water (20L), extracted three times with ethyl acetate (2L×3), combined the organic phases, followed by water (2L×5), saturated brine (2L), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 318 g of a yellow oil with a yield of 85.1%.
Embodiment 3
[0051] The preparation of embodiment 3 formula (V) compound
[0052] Add the compound of formula (IV) (280g, 0.6mol) and tetrahydrofuran (2.2L) into a 3L round-bottomed three-necked flask equipped with a drying tube. After stirring to dissolve, add 4N hydrochloric acid aqueous solution (300mL, 1.2mol), and continue the reaction at room temperature 2h, add 2L of water, concentrate under reduced pressure and distill THF off, extract three times with ethyl acetate (500mL×3), combine the organic phases, wash with water (500mL) and saturated brine (500mL) successively, dry over anhydrous sodium sulfate, filter , and concentrated to obtain 208 g of a yellow oil, with a yield of 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com