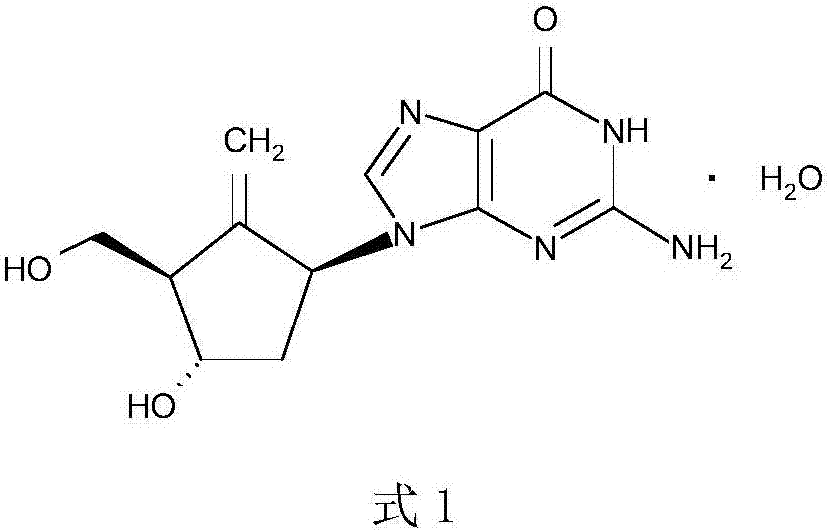
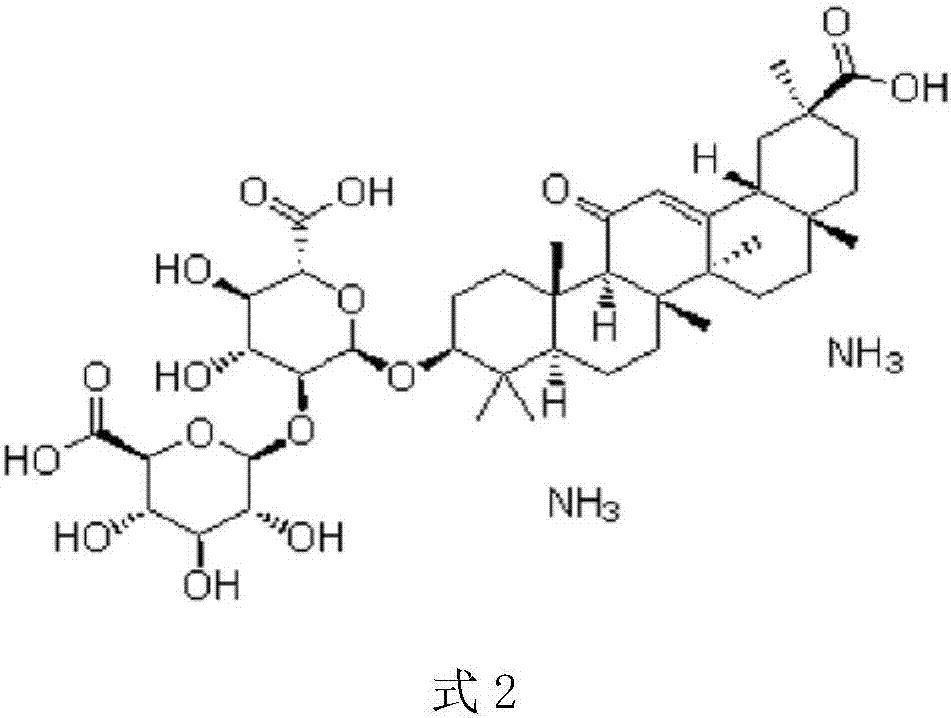
Compound pellets of diammonium glycyrrhizinate and entecavir and preparation method thereof
A technology of diammonium glycyrrhizinate and diammonium glycyrrhizinate intestine, which is applied in the field of medicine to achieve the effects of good uniformity, alleviating large differences in distribution and absorption in the body, and convenient simultaneous administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
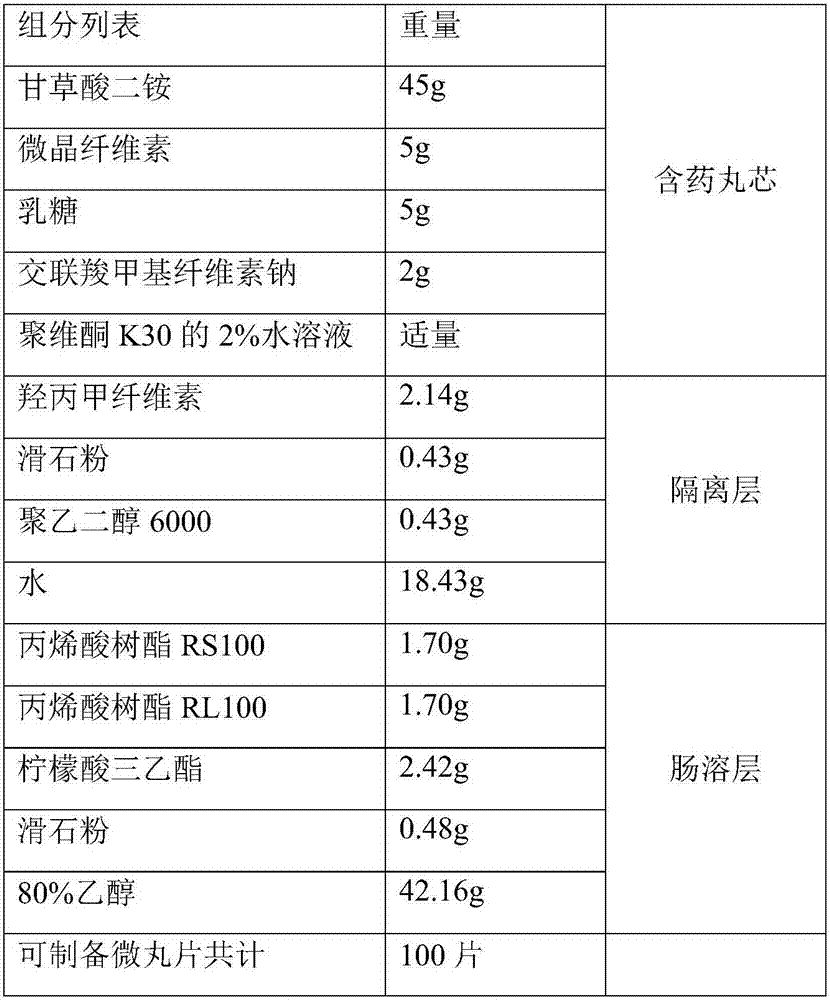
[0028] Embodiment 1: the preparation of diammonium glycyrrhizinate and entecavir compound pellets
[0029] 1) Preparation of diammonium glycyrrhizinate enteric-coated sustained-release pellets
[0030]
[0031] Remarks: The water in the above formulation is dried and removed during the preparation process.
[0032]Preparation method: Weigh the prescribed amount of raw materials and auxiliary materials, mix evenly, use 2% aqueous solution of povidone K30 as an adhesive to make soft materials, extrude and spheronize to prepare drug-containing pill cores, and sieve 24-30 meshes for use. Take the above-mentioned drug-containing pellet cores in a fluidized bed, prepare a coating solution and coat the isolation layer, and the weight of the coating increases by about 5%. Take the drug-containing pellets coated with the isolation coat, prepare an enteric coating solution to coat the enteric layer, and the weight of the coating increases by about 10%, to obtain diammonium glycyrrhi...
Embodiment 2
[0039] Embodiment 2: the preparation of diammonium glycyrrhizinate and entecavir compound pellets
[0040] 1) Preparation of diammonium glycyrrhizinate enteric-coated sustained-release pellets
[0041]
[0042] Remarks: The water in the above formulation is dried and removed during the preparation process.
[0043] Preparation method: Weigh the prescribed amount of raw materials and auxiliary materials, mix evenly, use 2% aqueous solution of povidone K30 as an adhesive to make soft materials, extrude and spheronize to prepare drug-containing pill cores, and sieve 24-30 meshes for use. Take the above-mentioned drug-containing pellet cores in a fluidized bed, prepare a coating solution and coat the isolation layer, and the weight of the coating increases by about 5%. Take the drug-containing pellets coated with the isolation coat, prepare an enteric coating solution to coat the enteric layer, and the weight of the coating increases by about 10%, to obtain diammonium glycyrrh...
Embodiment 3
[0051] Embodiment 3: the preparation of diammonium glycyrrhizinate and entecavir compound pellets
[0052] 1) Preparation of diammonium glycyrrhizinate enteric-coated sustained-release pellets
[0053]
[0054] Remarks: The water in the above formulation is dried and removed during the preparation process.
[0055] Preparation method: Weigh the prescribed amount of raw materials and auxiliary materials, mix evenly, use 2% aqueous solution of povidone K30 as an adhesive to make soft materials, extrude and spheronize to prepare drug-containing pill cores, and sieve 24-30 meshes for use. Take the above-mentioned drug-containing pellet cores in a fluidized bed, prepare a coating solution and coat the isolation layer, and the weight of the coating increases by about 5%. Take the drug-containing pellets coated with the isolation coat, prepare an enteric coating solution to coat the enteric layer, and the weight of the coating increases by about 10%, to obtain diammonium glycyrrh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com