A kind of binol derivative and its preparation method and application
A technology of derivatives and products, applied in the field of chemistry, can solve the problems of low recognition ability and small difference in slope ratio, and achieve fast and accurate analysis, less sample volume and good recognition effect
Active Publication Date: 2018-04-17
SOUTHWEST MEDICAL UNIVERISTY
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
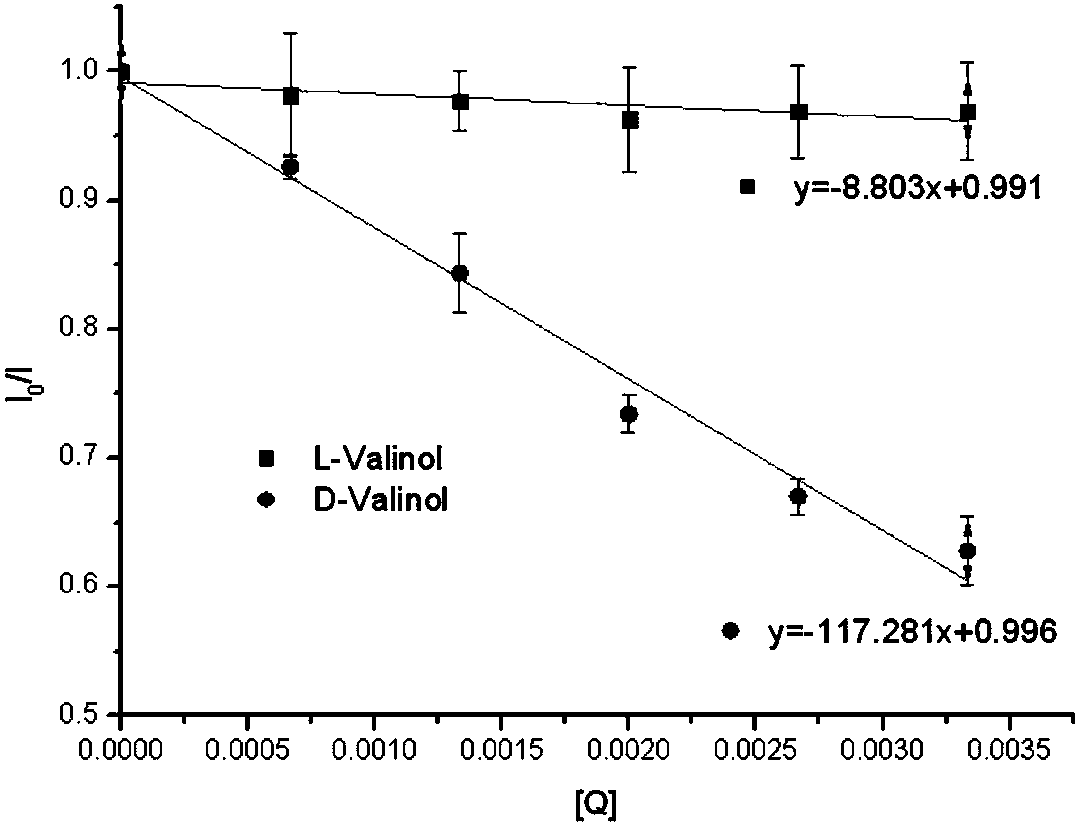
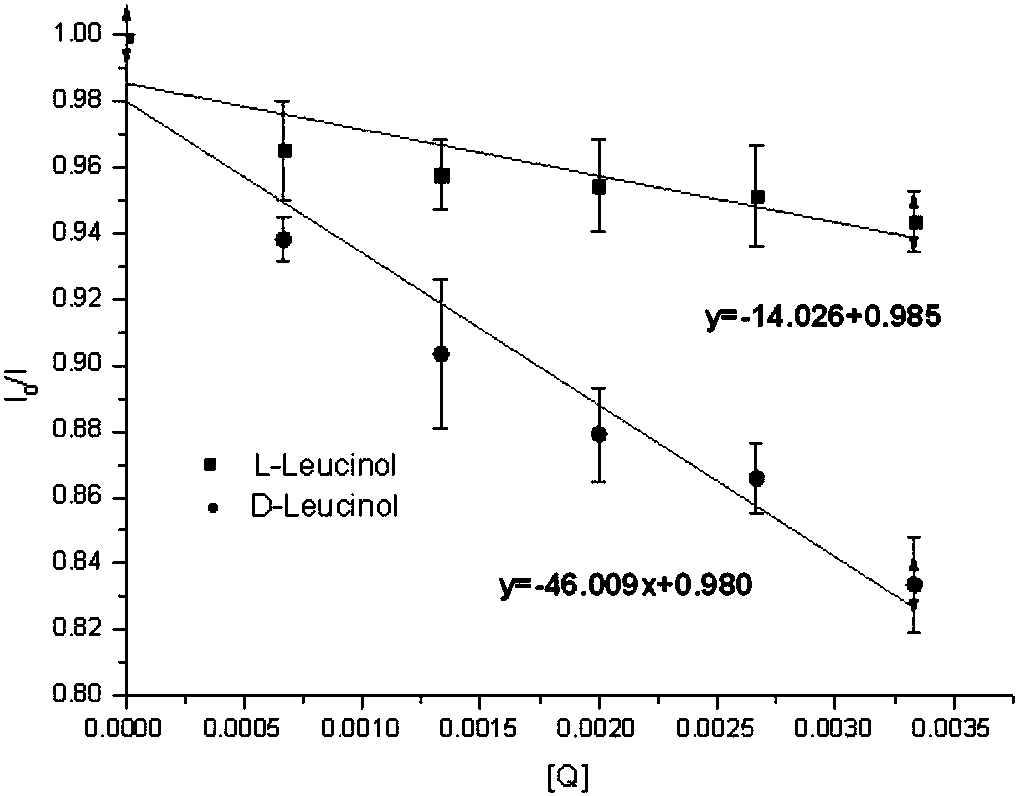
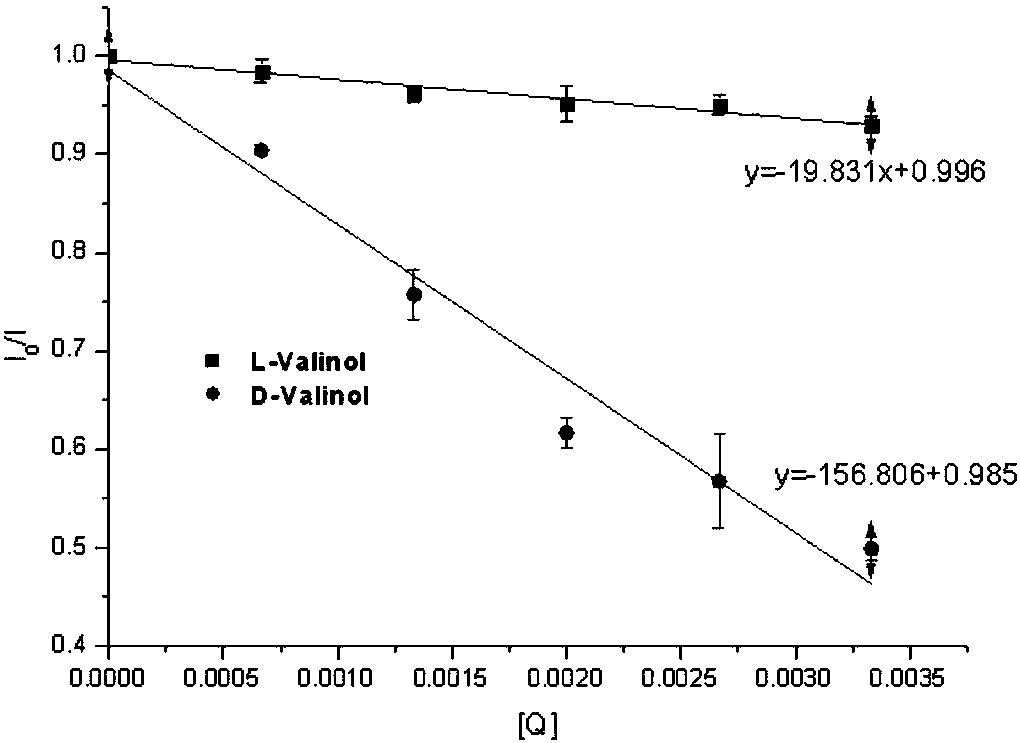
The commonly used 1,1'-bi-2-naphthol derivatives are C2 axisymmetric aromatic compounds, and the effect on amino alcohols is fluorescence quenching, and the ability to recognize the corresponding D-type and L-type amino alcohols lower, less difference in slope ratio
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0046] Embodiment 1: Sensor 1 having the following structure
[0047]((S)-3-(2-hydroxypropan-2-yl)-1,1'-binaphthyl-2,2'-diol)
[0048]
Embodiment 2
[0049] Embodiment 2: Sensor 2 having the following structure
[0050] ((S)-3-(3-hydroxypentan-3-yl)-1,1'-binaphthyl-2,2'-diol)
[0051]
Embodiment 3
[0052] Embodiment 3: sensor 3 having the following structure
[0053] ((S)-3-(hydroxydiphenylmethyl)-1,1'-binaphthyl-2,2'-diol)
[0054]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a BINOL derivative.The BINOL derivative has the R or S configuration of a compound represented as the general formula in the description, wherein R1 and R2 are alkyl groups or fluorine-containing phenyl groups independently.When R1 and R2 are the same and both methyl groups, ethyl groups or 3,5-bis (trifluoromethyl) phenyl, the BINOL derivative has a good chiral recognition effect.The BINOL derivative can quickly analyze and determine a chiral amino alcohol absolute configuration in real time.According to the method, the quantity of samples in use is small, analysis is quick and accurate, the sample structure is not damaged, configuration determination does not need a complex chemical reaction, the BINOL derivative has the advantage that fluorescence is enhanced along with increase of concentration of amino alcohol to be tested, and a recognition effect is good.
Description
technical field [0001] The invention belongs to the technical field of chemistry, and in particular relates to a BINOL derivative and a preparation method and application thereof. Background technique [0002] Enantioselective fluorescence recognition technology can provide real-time rapid analytical detection for the determination of the absolute configuration of chiral organic compounds, and is used to determine the absolute configuration of chiral amino alcohols. The method uses a small amount of sample, is fast and accurate in analysis, and has no damage to the sample structure. Photoactive 1,1′-bi-2-naphthol derivatives have strong chiral induction, and can produce strong fluorescence under the modification of suitable structural groups, which can be used as fluorescent chemical sensors and chiral amines, amino groups Alcohols, amino acids, α-hydroxy carboxylic acids, chiral alcohols, and monosaccharides act on chiral organic compounds to accept guest molecules at diff...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C39/14C07C39/38C07C37/055
CPCC07C37/055C07C41/30C07C39/14C07C39/38C07C43/23
Inventor 王力王钦韦思平尤强刘大亮彭瑞光杜曦
Owner SOUTHWEST MEDICAL UNIVERISTY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com