A method for determining the content of oligosaccharides in compound salvia miltiorrhiza extract
A technology of compound salvia miltiorrhiza and its determination method, which is applied in the detection field of active ingredients of traditional Chinese medicine, and can solve problems such as low sensitivity and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Determine according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 edition).
[0088] Chromatographic conditions and system suitability test
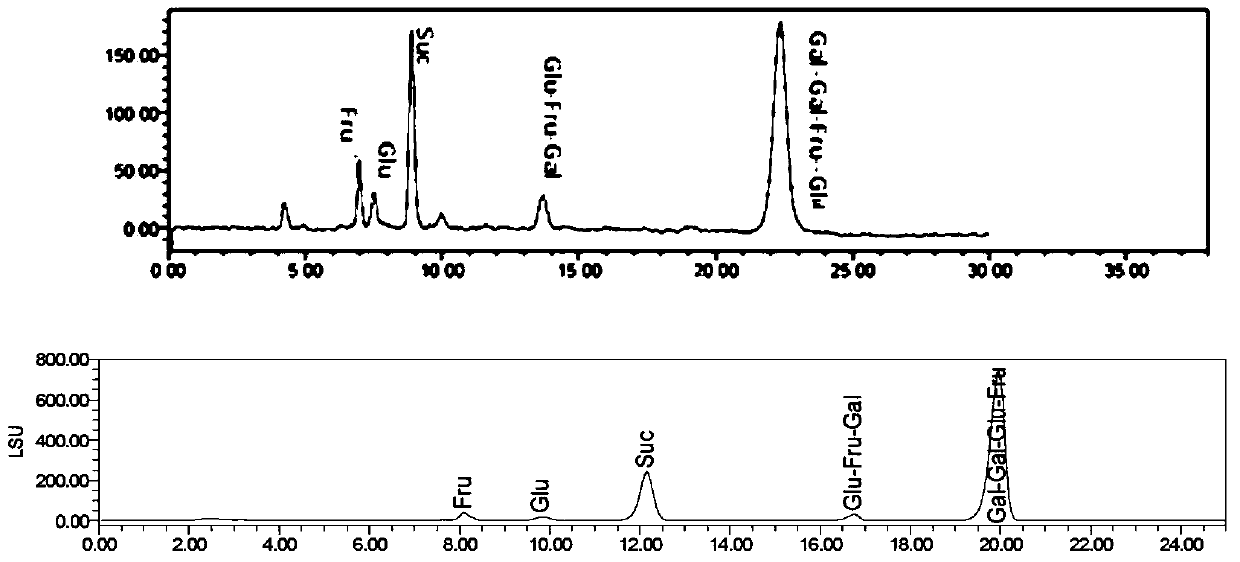
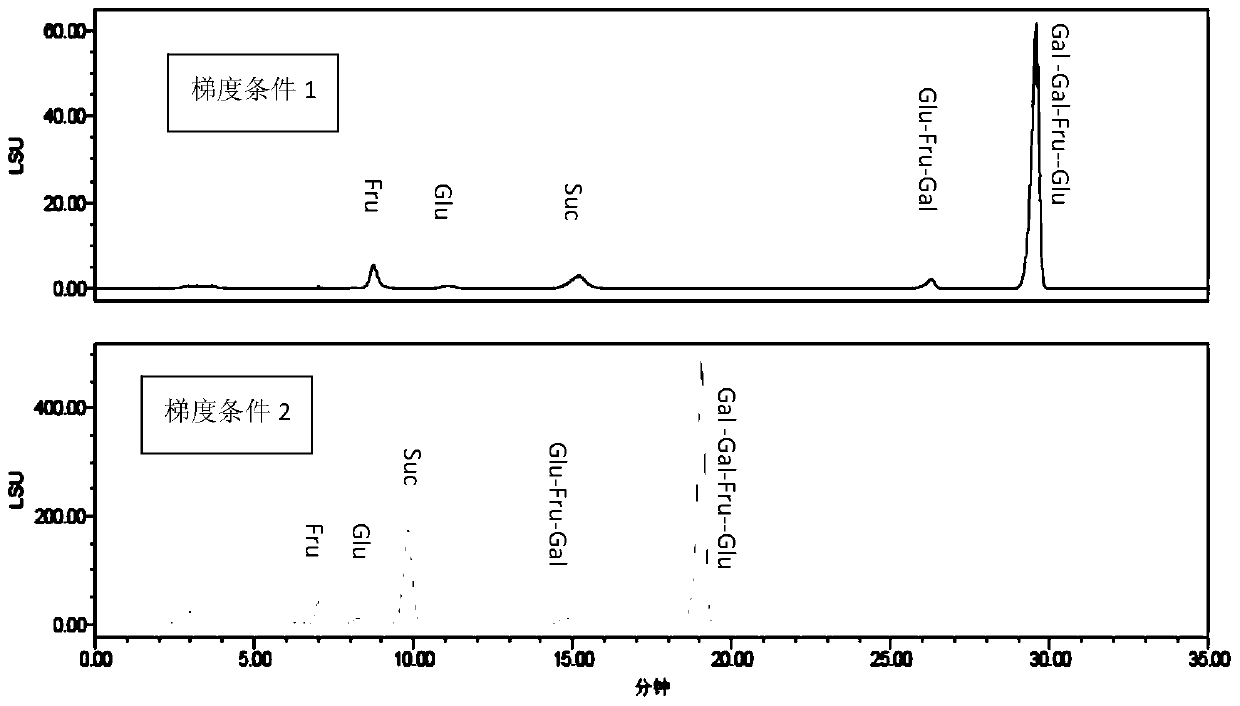
[0089] PrevailTM Carbohydrate ES chromatographic column was used, with acetonitrile as mobile phase A, 0.1% formic acid water as mobile phase B, flow rate 0.8ml / min, column temperature 30°C; WATERS 2420ELSD detector was used for detection, parameters were set to gain 10, air pressure 30psi , drift tube 60°C, Neb heater: 60%. The number of theoretical plates calculated based on the sucrose chromatographic peak should not be less than 4000, and the chromatographic resolution of fructose and glucose should be greater than 2.0.
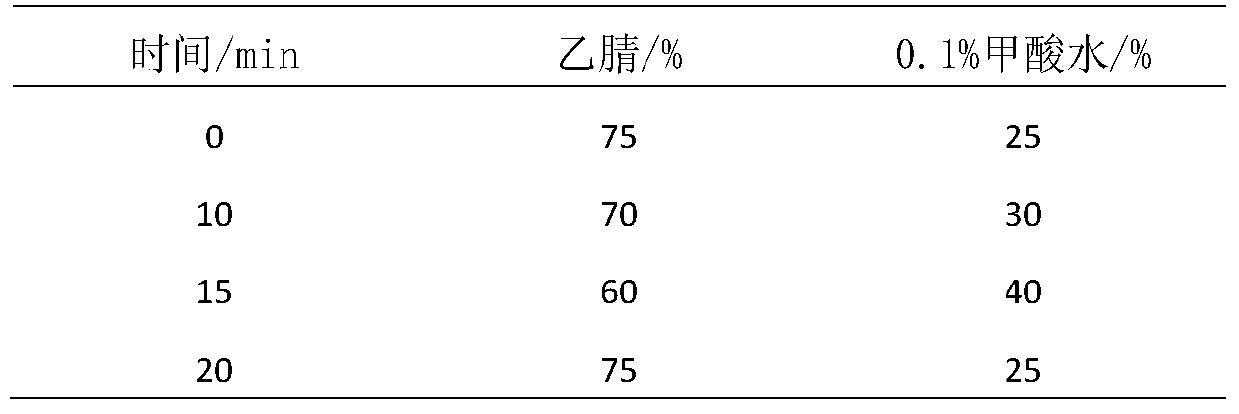
[0090] time (minutes) Acetonitrile 0.1% formic acid in water 0 75 25 10 70 30 15 60 40 20 75 25
[0091] Preparation of reference substance solution Accurately weigh an appropriate amount of fructose, glucose, sucrose, raffin...
Embodiment 2
[0095] Measure according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2010 edition).
[0096] Chromatographic conditions and system suitability test
[0097] PrevailTM Carbohydrate ES chromatographic column was used, with acetonitrile as mobile phase A, 0.1% formic acid water as mobile phase B, flow rate 0.8ml / min, column temperature 30°C; WATERS 2420ELSD detector was used for detection, and the parameters were set to gain 10, air pressure 25psi, drift tube 65°C, Neb heater: 60%. The number of theoretical plates calculated based on the sucrose chromatographic peak should not be less than 4000, and the chromatographic resolution of fructose and glucose should be greater than 2.0.
[0098] time (minutes) Acetonitrile 0.1% formic acid in water 0 75 25 10 70 30 15 60 40 20 75 25
[0099] Preparation of reference substance solution Accurately weigh an appropriate amount of fructose, glucose, sucrose, ra...
Embodiment 3
[0103] Determine according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 edition).
[0104] The chromatographic conditions and system suitability test used PrevailTM Carbohydrate ES chromatographic column, with acetonitrile as mobile phase A, 0.2% formic acid water as mobile phase B, flow rate 0.85ml / min, column temperature 30°C; detection by WATERS 2420ELSD detector, The parameters are set to gain 10, air pressure 30psi, drift tube 60°C, Neb heater: 60%. The number of theoretical plates calculated based on the sucrose chromatographic peak should not be less than 4000, and the chromatographic resolution of fructose and glucose should be greater than 2.0.
[0105] time (minutes) Acetonitrile 0.1% formic acid in water 0 75 25 10 70 30 15 60 40 20 75 25
[0106] Preparation of reference substance solution Accurately weigh an appropriate amount of fructose, glucose, sucrose, raffinose and Gal-Gal-G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com