Mammalian cell culture technology for enhancing monoclonal antibody ADCC activity
A monoclonal antibody, mammalian technology, applied in the field of mammalian cell flow plus culture process and mammalian cell culture process, can solve the problems affecting the safety and effectiveness of monoclonal antibodies, produce immunogenicity, and accelerate antibody clearance. Achieve the effect of improving quality, improving biological function, and improving ADCC activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 adds the shake flask culture of different concentration uridine and manganese sulfate
[0037] Shake flask culture cells were seeded at a density of 0.8-1.2×10 6 cells / mL, working volume 50mL, shaker (Konai) control: speed is 120-130rpm, temperature is 36.5°C, CO2 saturation is 7%, additional feed is added on the 3rd, 5th, 7th, 9th and 11th day of culture culture medium and different total amounts of manganese sulfate and uridine), and the culture period is 13 days. After the incubation, the protein A purified antibody was used for quality analysis.
[0038] Detection method:
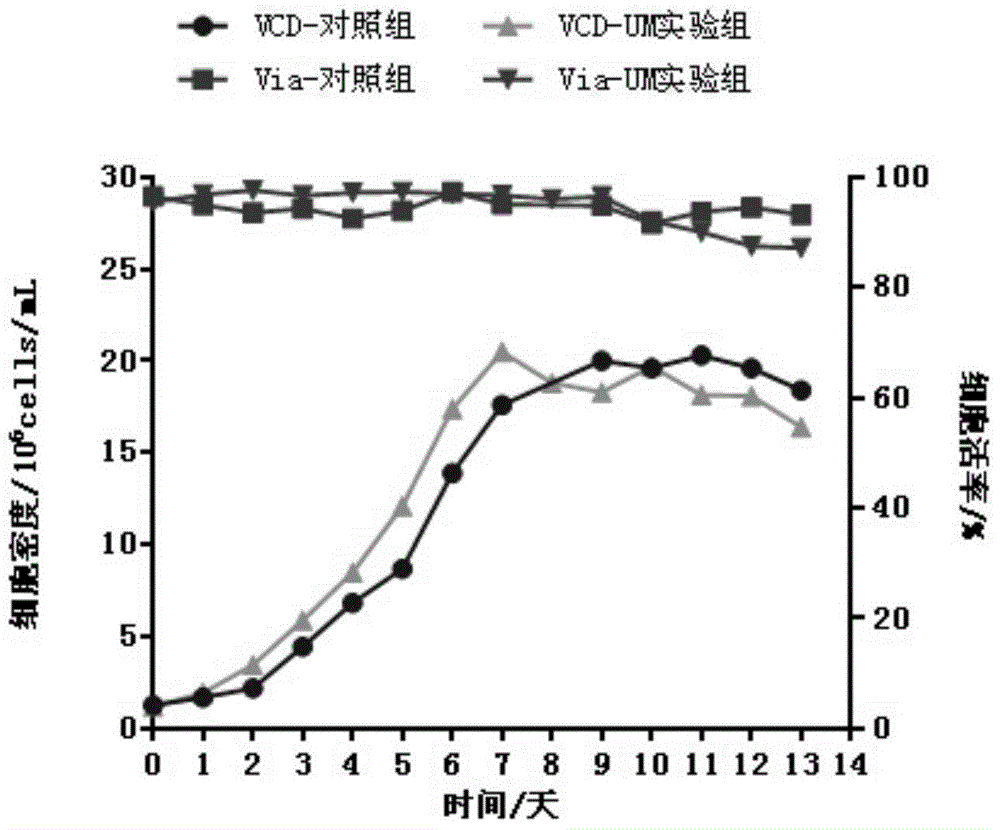
[0039] Detection of viable cell density: Counterstar was used to detect the density of viable cells, and the results are shown in Table 1.
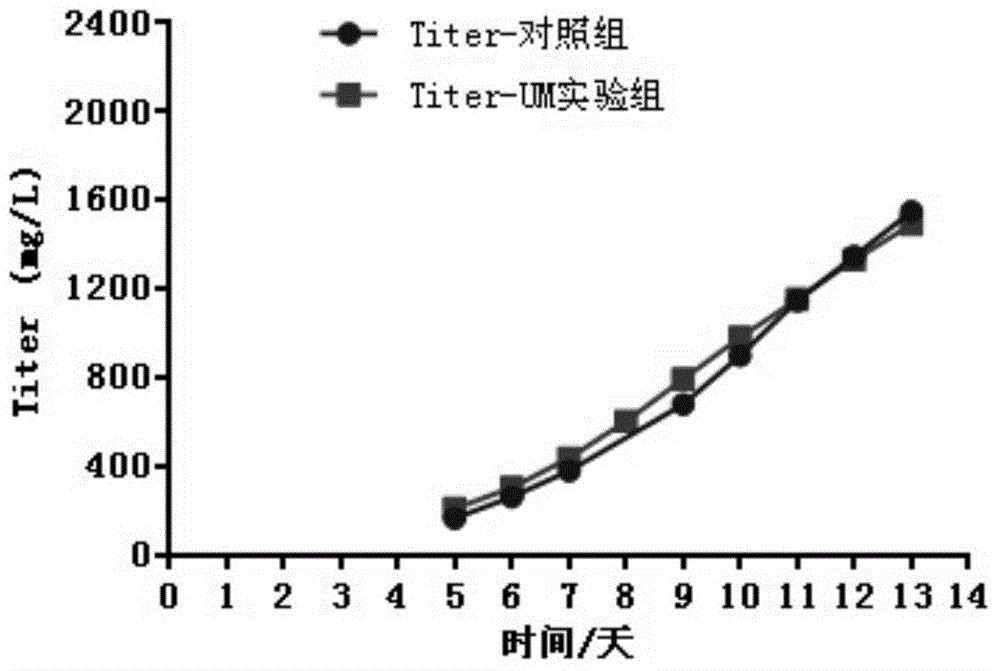
[0040] Antibody titer detection: The antibody titer was detected by HPLC-Protein A method, and the results are shown in Table 1.
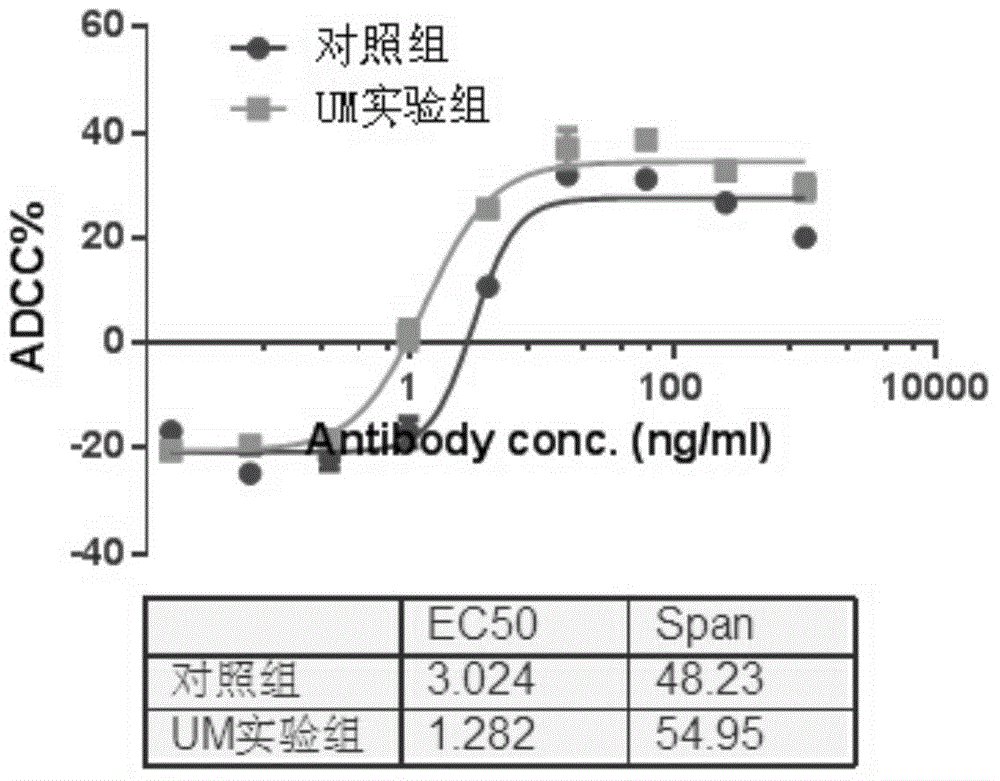
[0041] ADCC activity detection: engineered NK92-CD16a cells were used as effector cells, and Wil2-s cells were...
Embodiment 23L
[0049] Embodiment 23L reactor process test
[0050] The cells were inoculated into a 3L glass reactor (Applikon, Netherlands) for process testing after being expanded in shake flasks, and the cell inoculation density was 0.8-1.2×10 6 cells / mL, the control parameters are: the stirring speed is 80-250rpm, the temperature is 36.5°C, the pH is 7.0, the dissolved oxygen is 40% (air saturation), and the fed-batch medium containing The fed-batch medium of uridine and manganese sulfate has a culture period of 13 days, the total fed-batch amount of uridine is 8mM, and the total feed-batch amount of manganese ions is 16μM. In the control group, only fed-batch medium was added. During the culture, samples were taken every day for growth, metabolism and titer detection. After culturing, antibodies were purified by affinity chromatography, cation exchange chromatography and anion exchange chromatography for quality analysis.
[0051] Detection method:
[0052] Viable cell density detec...
Embodiment 3250
[0063] Embodiment 3250LSUB reactor cultivation process verification
[0064] At the 250L level, repeat 3 batches of Chinese-style scale-up culture.
[0065] Thermo250LSUB reactor culture: cell seeding density is 0.8-1.2×10 6 cells / mL, the control parameters are: the stirring speed is 80-120rpm, the temperature is 36.5°C, the pH is 6.9-7.1, the dissolved oxygen is 30-50% (air saturation), the initial working volume is 150L, and at the 3rd-4th day of culture , add fed-batch medium (containing uridine and manganese ions), maintain the glucose concentration to 2g / L, the culture period is 14-16 days, and finally contain 8mM uridine and 16μM in the culture solution.
[0066] Such as Figure 4 and Figure 5 , the growth and expression of the three batches of 250LSUB reactors were consistent, and the EC50 of ADCC of the expressed products were 1.189ng / mL, 1.449ng / mL and 1.362ng / mL, CV<10%, with good reproducibility.
[0067] The fed-batch culture process of adding amino acid and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com